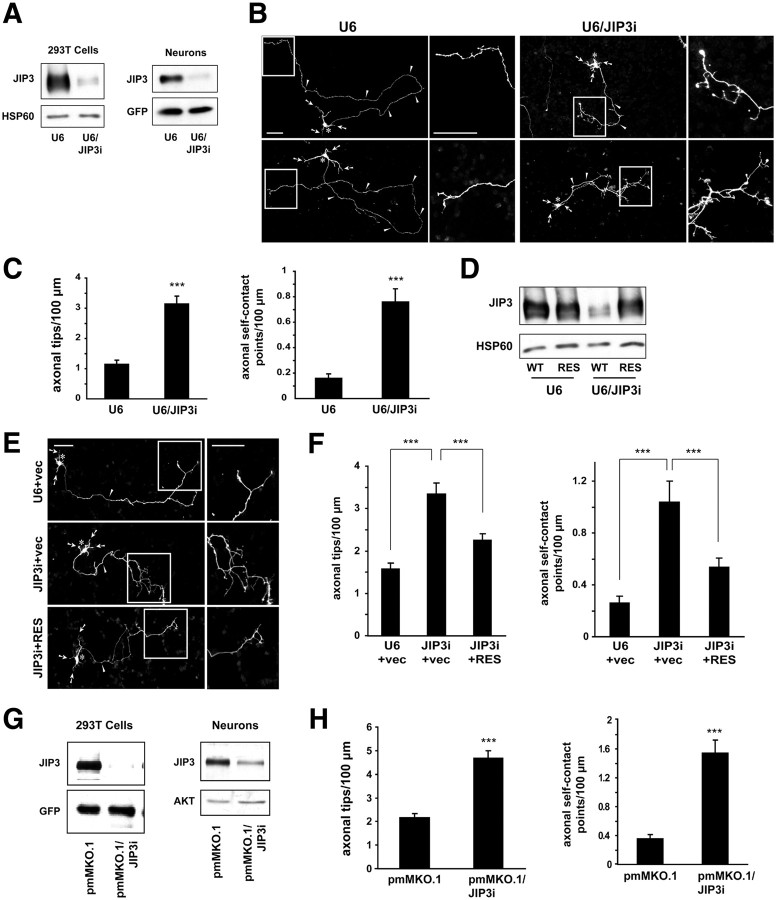
Figure 1.
JIP3 restricts axon branching in cerebellar granule neurons. A, Left, Lysates of HEK293T cells transfected with the FLAG–JIP3 expression plasmid and the U6/JIP3i or control U6 RNAi plasmid were immunoblotted with the FLAG and HSP60 antibodies, the latter serving as a loading control. Right, Lysates of granule neurons nucleofected with the U6/JIP3i or control U6 RNAi plasmid together with a GFP expression plasmid were immunoblotted using the JIP3 and GFP antibodies, the latter serving as a control for loading and transfection efficiency. B, Granule neurons transfected with the U6/JIP3i or control U6 RNAi plasmid together with the GFP expression plasmid were fixed 4 d after transfection and subjected to immunocytochemistry using the GFP antibody. For each representative image, boxed regions in the left panel highlight the distal axon, which is shown at higher magnification in the right panel. In all images of this type, arrows indicate dendrites, asterisks the soma, and arrowheads the axon. Scale bars, 50 μm. JIP3 knockdown triggered the formation of exuberant axon branches. C, Quantification of axon branching of granule neurons treated as in B. JIP3 knockdown significantly increased the number of axonal tips per hundred micrometers (t test, p < 0.001) and the number of axonal self-contact points per hundred micrometers (t test, p < 0.001). A total of 160 neurons were measured. Error bars here and in all subsequent bar graphs denote +SEM. Except when indicated otherwise, in all figures asterisks on bar graphs denote statistical significance compared with the control condition: *p < 0.05, **p < 0.01, and ***p < 0.001. D, Lysates of HEK293T cells transfected with FLAG–JIP3 encoded by wild-type cDNA (WT) or an RNAi-resistant cDNA (RES) and the U6/JIP3i or control U6 RNAi plasmid were immunoblotted using the FLAG and HSP60 antibodies. JIP3 knockdown decreased levels of FLAG–JIP3 but not FLAG–JIP3–RES. E, Granule neurons transfected with the FLAG–JIP3–RES or control vector expression plasmid and the U6/JIP3i or control U6 RNAi plasmid together with the GFP expression plasmid were analyzed as in B. Scale bars, 50 μm. F, Quantification of axon branching of granule neurons treated as in E. In the vector background, JIP3 knockdown significantly increased the number of axonal tips per hundred micrometers (ANOVA followed by Fisher's PLSD test, p < 0.001) and the number of axonal self-contact points per hundred micrometers (ANOVA followed by Fisher's PLSD test, p < 0.001). In the background of JIP3 knockdown, expression of FLAG–JIP3–RES, compared with the corresponding vector, significantly decreased the number of axonal tips per hundred micrometers (ANOVA followed by Fisher's PLSD test, p < 0.001) and the number of axonal self-contact points per hundred micrometers (ANOVA followed by Fisher's PLSD test, p < 0.001). A total of 225 neurons were measured. G, Left, Lysates of HEK293T cells transfected with the FLAG–JIP3 expression plasmid and the pmMKO.1/JIP3i or control pmMKO.1 RNAi plasmid were immunoblotted using the FLAG and GFP antibodies. Right, Lysates of granule neurons nucleofected with the pmMKO.1/JIP3i or control pmMKO.1 RNAi plasmid were immunoblotted using the JIP3 and AKT antibodies, the latter serving as a loading control. H, Quantification of axon branching of granule neurons transfected with the pmMKO.1/JIP3i or control pmMKO.1 RNAi plasmid together with the GFP expression plasmid, analyzed as in B. Knockdown of JIP3 with this second hairpin increased the number of axonal tips per hundred micrometers (t test, p < 0.001) and the number of axonal self-contact points per hundred micrometers (t test, p < 0.001). A total of 156 neurons were measured.