Figure 3. CCL2 promotes Src-dependent FAK activation and stress fiber formation in retracting EC.
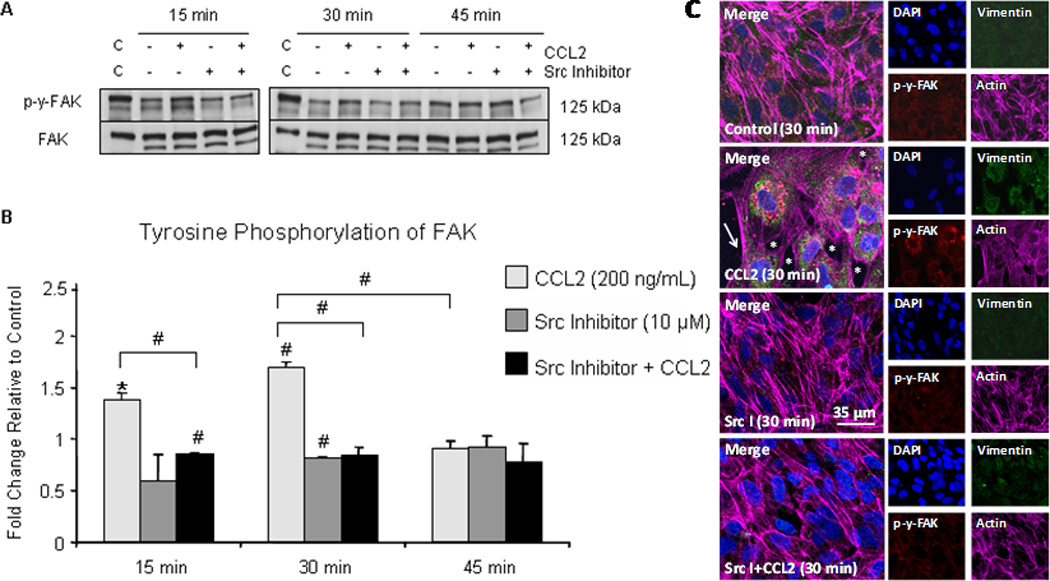
(A) hCMEC/D3 were grown to confluence on gelatin-coated tissue culture dishes and pre-treated with 10 µM Src inhibitor or DMSO for 2 hours followed by treatment with 200 ng/mL CCL2 or diluent. Cells were lysed and 40 µg of total protein was analyzed by Western blot. Blots were first evaluated with p-Y-576/p-Y-577-FAK antibody, followed by stripping of phospho-specific antibody and re-probing with antibody to total FAK. Figure 3A is a representative blot of 3 independent experiments. C = control lysate. (B) Optical density (OD) of tyrosine phosphorylated FAK was evaluated relative to OD of non-phosphorylated FAK. Densitometry is reported as fold change in tyrosine phosphorylated FAK in response to CCL2 relative to control at same time point. n=3. (#) p < 0.01. FAK activation, as evident by phosphorylation, is increased at 15 minutes and 30 minutes and is reduced with Src inhibitor pre-treatment. By 45 minutes, the level of FAK activation is equal to control. (C) Primary HBMVEC were grown to confluence on gelatin-coated MatTek dishes. Following Src inhibitor pre-treatment, cells were treated with 200 ng/mL CCL2 or diluent. Cells were fixed, permeabilized, and immunofluorescently stained with DAPI (blue)- nucleus, Cy3 (red)- p-y-FAK, FITC (green)- vimentin, and Cy5 (magenta)- actin. Cells treated with CCL2 demonstrate increased p-y-FAK staining in retracting cells relative to control cells. Interendothelial gaps in monolayer (asterisk) and EC stress fiber formation (arrow) are evident. Pre-treatment with Src inhibitor abrogates FAK phosphorylation and formation of interendothelial gaps and EC stress fibers.
