Abstract
The sequestrate fungi of Japan, including truffle and truffle-like fungi, have not been well characterized but are potentially diverse. We investigated the diversity and phylogeny of Japanese Octaviania specimens using a multifaceted approach including scanning and transmission electron microscopy as well as analysis of nuclear ribosomal DNA (ITS and LSU) and EF-1α (tef1) sequences. Phylogenetic analyses indicate that the genus Octaviania is divided into three major clades, and that there are at least 12 species-level lineages in Japan. Accordingly, we describe two new subgenera, Parcaea and Fulvoglobus, and eleven new species. Subgenus Parcaea accommodates four highly divergent, but macromorphologically almost indiscernible cryptic species. We discuss not only the diversity and species delimitation within the genus Octaviania but also the phylogeography of the Japanese taxa and their relatives.
Keywords: biogeography, Boletaceae, cryptic species, hypogeous fungi, phylogeny, taxonomy
INTRODUCTION
Species in the sequestrate, truffle-like genus Octaviania Vittad. (orthographic variant: Octavianina Kuntze (Gams 1999); Boletaceae, Boletales) are characterized by a marbled gleba and dextrinoid or non-amyloid basidiospores with coarse, conical to pyramidal ornamentation. Like many other sequestrate fungi, Octaviania species form ectomycorrhizas with woody plants (Chu-Chou & Grace 1983, Frank et al. 2006). Vittadini erected Octaviania in 1831 but the genus was broadly interpreted by subsequent authors, resulting in a chaotic taxonomy with competing generic concepts (e.g., Lloyd 1922, Zeller & Dodge, 1936, Cunningham 1944, Cribb 1958). Pegler & Young (1979) thoroughly revised the genus and clarified the taxonomic concepts within the group, but the true diversity within Octaviania is still unresolved. Historically, nearly 100 infrageneric taxa have been described at one time or another in Octaviania. Many of them were transferred to other sequestrate genera such as Arcangeliella, Gymnomyces, Hydnangium, Melanogaster, and Stephanospora (e.g., Pegler & Young 1979, Trappe et al. 2002, Vidal 2004), and some others were synonymized into different Octaviania species (Lenne 2005). As a result, there are only approximately 15–20 accepted species that fit the current generic concept of the genus.
The evolutionary relationships of the genus with other Agaricomycetes have long been uncertain because of their unique morphology (Thiers 1984). Recent molecular studies of the Boletales have placed Octaviania within the Boletaceae (Binder & Hibbett 2006), but the infrageneric systematics of Octaviania has not yet been studied. The well-known type species, O. asterosperma Vittad. has been reported worldwide (e.g., Vittadini 1831, Massee 1889, Coker & Couch 1928, Zeller & Dodge 1936, Hawker 1954, Moreno et al. 1991, Cázares et al. 1992, Tao et al. 1998, Montecchi & Sarasini 2000), but morphological diversity among collections from different regions indicates that this may not be a truly cosmopolitan species. Intensive studies in one region using both morphological and molecular techniques may reveal greater species diversity of the genus and provide a more precise understanding of its biogeography.
Three Octaviania species have so far been reported from Japan: O. columellifera Kobayasi (Kobayasi 1937), O. asterosperma (Kobayasi et al. 1987, Yoshimi & Doi 1989), and O. tuberculata Hesse (Yoshimi & Doi 1989). However, a recent study by Orihara et al. (2010) revealed that O. asterosperma sensu Yoshimi & Doi was a misidentification of O. columellifera, and they excluded O. columellifera from Octaviania and placed it into a new genus, Heliogaster. Another doubtful record of O. asterosperma from Japan was based on an insufficiently described specimen (Kobayasi et al. 1987) and is treated here as Octaviania sp.
The only remaining Japanese Octaviania species, O. tuberculata, was collected in a subtropical angiosperm forest in Amami-oshima Island in the Ryukyu Archipelago (Yoshimi & Doi 1989) and has not been subsequently reported. There are crucial morphological differences between the Japanese O. tuberculata described by Yoshimi & Doi (1989) and the authentic O. tuberculata from Europe, suggesting that this species report should be re-examined. Our recent intensive collecting of Japanese sequestrate fungi has yielded many unique fruitbodies that do not fit descriptions of any known Octaviania species, suggesting that the genus has greater diversity in this region than expected.
The aims of this study are: 1) clarify the diversity and the phylogenetic relationships of Octaviania in Japan; and 2) taxonomically evaluate each resulting species using a comprehensive approach that includes morphological, molecular, ecological, and phylogeographic evidence. We therefore conducted phylogenetic analyses and molecular comparison using the ITS1-5.8-ITS2 (ITS) region and large subunit (LSU) of nuclear rDNA (nrDNA) and the elongation factor-1α (EF-1α or tef1) gene datasets as well as macro- and micromorphological comparative observations including scanning electron microscopy (SEM) and transmission electron microscopy (TEM). In this publication we present a key to all known Japanese Octaviania species and we propose 11 new Octaviania species and two new subgenera (Parcaea and Fulvoglobus). We also propose a substitute name for O. nigrescens (Zeller) Singer & A.H. Sm., which is a later homonym of O. nigrescens J.W. Cribb.
MATERIALS AND METHODS
Taxon sampling and macro- and microscopic characterization
Fresh basidiomata were collected at various locations throughout Japan. After macro- and micromorphological observation and DNA extraction, the collected basidiomata were air-dried or freeze-dried for later examination. Dried herbarium specimens were obtained from the National Museum of Nature and Science, Tokyo (TNS), Kanagawa Prefectural Museum of Natural History (KPM), the Oregon State University Herbarium, Oregon, USA (OSC), the New York Botanical Garden, New York, USA (NY), and the Royal Botanic Gardens, Melbourne, Australia (MEL). The Octaviania specimens collected from Japan were grouped into 9 lineages (i.e., the Octaviania lineages A–I) based on morphological and ecological features. For standard light microscopy and differential interference contrast (DIC) microscopy, hand-cut sections of both fresh and dried specimens were mounted in water, 3 % KOH, lactic acid, lactoglycerol, lactophenol-cotton blue (LCB), or 1 % phloxine B aqueous solution. Ectomycorrhizas of Octaviania species were collected and examined when they were connected to basidiomata via rhizomorphs. Colours are described in general terms. Spore and spore ornament dimensions were measured based on 50 randomly selected spores unless otherwise noted. Since Orihara et al. (2010) noted that Octaviania basidiospore morphology (including spore ornament dimensions) is easily affected by acidic or alkaline solutions, the basidiospores and their ornaments were measured in water. The measurement was done using ImageJ v. 1.4.3 (http://rsbweb.nih.gov/ij/) and excluded both ornamentation and hilar appendages. The 95 % prediction interval of diameter of the basidiospores, shown without parentheses, was calculated as: (the endpoints of the 95 % prediction interval) = M ± t · SD 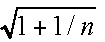 (where M = mean value; t = t value under a Student’s t-distribution with n-1 degrees of freedom (P = 0.05); SD = standard deviation; n = 50). The actual range in the measurement is shown in parentheses, but is omitted when it is the same as the value of the 95% prediction interval. Average size of basidia was calculated based on 15 randomly selected basidia unless otherwise noted.
(where M = mean value; t = t value under a Student’s t-distribution with n-1 degrees of freedom (P = 0.05); SD = standard deviation; n = 50). The actual range in the measurement is shown in parentheses, but is omitted when it is the same as the value of the 95% prediction interval. Average size of basidia was calculated based on 15 randomly selected basidia unless otherwise noted.
SEM was carried out according to Maekawa (1987). TEM protocols for fixation, post-fixation, dehydration, resin impregnation, and observation were those of Shimomura et al. (2007). Specimens examined in this study were deposited in TNS or KPM.
DNA extraction, PCR amplification and sequencing
DNA was extracted from fresh or dried basidiomata using the FTA Classic Card or the Indicating FTA Cards (Whatman International Ltd, Maidstone, England) according to the manufacturer’s instructions. PCR amplification of the ITS region and a part of the nLSU of the rDNA was carried out using one or two prepared FTA discs 2 mm diam, Ampdirect Plus and Nova Taq (Shimadzu Corporation, Kyoto, Japan) according to the manufacturer’s instructions. The primer pairs for PCR were ITS1F (Gardes & Bruns 1993) / ITS4 (White et al. 1990) or ITS4B (Gardes & Bruns 1993), ITS1F / ITS2 (White et al. 1990), and/or ITS3 (White et al. 1990) / ITS4F for the ITS region, LR0R / LR5 (Vilgalys & Hester 1990) for the nLSU, and EF1-983F / EF1-2218R or EF1-1567R (Rehner & Buckley 2005) for EF-1α. Reactions were performed on a Astec Program Temp Control System PC-812 (Astec, Fukuoka, Japan) as follows: initial incubation at 95 °C for 10 min; in ITS amplification, a subsequent step of 10–12 cycles at 94 °C for 30 s, 60 °C for 60 s (decreasing 1 °C per cycle in the last 5 cycles), and 72 °C for 60 s (extending 1 s per cycle in the last 5 cycles), followed by 25 cycles at 94 °C for 30 s, 55 °C for 60 s, and 72 °C for 66 s (extending 1 s per cycle; thus the final elongation time is 90 s); in nLSU amplification, 35 cycles at 94 °C for 30 s, 50 °C for 60 s, and 72 °C for 90 s (extending 1 s per cycle in the last 30 cycles), followed by 5 cycles at 94 °C for 30 s, 50 °C for 60 s, and 72 °C for 120 s; and in EF-1α, 10 cycles at 94 °C for 30 s, 65 °C for 60 s (decreasing 1 °C per cycle), and 72 °C for 90 s, followed by 30 cycles at 94 °C for 30 s, 55 °C for 60 s, and 72 °C for 91 s (extending 1 s per cycle; thus the final elongation time is 90 s). A final elongation step of each run was at 72 °C for 7 min. All amplified PCR products were cleaned with ExoSAP-IT (USB Corporation, Cleveland, Ohio, USA) using the manufacturer’s instructions. Bidirectional sequencing of the PCR products was performed using BigDye v. 3.1 terminator cycle sequencing kit (Applied Biosystems, Foster City, California, USA) with the following protocol: initial denaturation at 94 °C for 1 min, 94 °C for 10 s (denaturation), 55 °C (ITS) or 50 °C (nLSU and EF-1α) for 5 s (annealing), and 60 °C for 4 min (elongation). Primer pairs used for sequencing reactions were the same as above for ITS and nLSU rDNA whereas for EF-1α sequencing the primer EF1-2212R was used instead of EF1-2218R. Sequencing was conducted on an ABI 3130 Genetic Analyser or an ABI 3730xl DNA Analyser (Applied Biosystems, Foster City, California, USA). Sequences were edited with Sequence Scanner v. 1.0 (Applied Biosystems, Foster City, California, USA), BioEdit v. 7.0.9 (Hall 1999), and Clustal X v. 1.83 (Thompson et al. 1997).
Phylogenetic analysis
Specimens used for phylogenetic analyses are listed in Table 1 and 2. A total of 77 sequences identified to the species level, including 12 sequences from GenBank/DDBJ/EMBL, were used for nLSU analyses. Sequences of Tylopilus chromapes (JN378517) and Austroboletus spp. (Table 2) were used as outgroups for the Octaviania-Leccinum-Leccinellum-Chamonixia-Rossbeevera clade. GenBank sequences of Octaviania nigrescens (HQ328787–HQ328788) and O. tasmanica (HQ328790) were identical to those used in this study, but they were considerably shorter (< 624 bp long) and were thus not used in the analyses. For EF-1α analyses, a total of 56 sequences were included and T. chromapes JN378457 was selected as the outgroup taxon. For the combined nLSU and EF-1α phylogeny, a total of 65 sequences identified to the species level were selected based on BLAST search and downloaded from GenBank. For the ITS phylogenetic network analyses, sequences of O. asterosperma EU784378–EU784379 and Octaviania sp. EU414289–EU414290 were retrieved from the database. Multiple sequence alignment was performed using Clustal X and the data were manually adjusted in SeaView (Galtier et al. 1996). Gaps were treated as ‘missing’ data for all analyses. All alignments and Bayesian trees generated from each alignment are deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S11908).
Table 1.
Newly obtained sequences of Octaviania, Leccinum, Leccinellum, Chamonixia, Rossbeevera, Turmalinea and outgroup species. The nLSU sequences excluded from the combined analyses are indicated as asterisks (*). The nLSU sequence of Rossbeevera eucyanea (HQ693879) was retrieved from GenBank (**).
| Taxon | Locality | Voucher No. | GenBank No. |
||
|---|---|---|---|---|---|
| ITS | nLSU | EF-1α | |||
| Octaviania lineage A-1 | Japan, Kagoshima Pref., Amami-oshima Isl. | KPM-NC-0017748 | JN257985 | JN378459 | JN378403 |
| Octaviania lineage A-1 | Japan, Kagoshima Pref., Amami-oshima Isl. | KPM-NC-0017749 | JN257986 | JN378460 | JN378404 |
| Octaviania lineage A-1 | Japan, Mie Pref., Kameyama-shi | KPM-NC-0017750 | JN257987 | JN378461 | JN378405 |
| Octaviania lineage A-1 | Japan, Hyogo Pref., Shiogahara | KPM-NC-0017751 | N257988 | JN378462 | JN378406 |
| Octaviania lineage A-1 | Japan, Hiroshima Pref., Hiroshima-shi, Higashi-ku, | KPM-NC-0017752 | JN257989 | JN378463 | JN378407 |
| Hiroshima Prefecture Ryokka-Center | |||||
| Octaviania lineage A-1 | Japan, Oita Pref., Saiki-shi, Shiroyama | KPM-NC-0017745 | JN257990 | JN378464 | JN378408 |
| Octaviania lineage A-2 | Japan, Kyoto Pref., Mt Hiei | KPM-NC-0017763 | JN257991 | JN378465 | JN378409 |
| Octaviania lineage A-2 | Japan, Kyoto Pref., Mt Ponpon | KPM-NC-0017764 | JN257992 | JN378466 | JN378410 |
| Octaviania lineage A-2 | Japan, Aichi Pref., Okazaki-shi, Ohata-cho, Nishiyama | KPM-NC-0017765 | – | JN378467* | – |
| Octaviania lineage A-2 | Japan, Oita Pref., Saiki-shi, Ume-Oaza, Shigeoka | KPM-NC-0017767 | JN257993 | JN378468 | JN378411 |
| Octaviania lineage A-2 | Japan, Oita-shi, Oaza, Hisado, Yayama | KPM-NC-0017768 | JQ619186 | JN378469 | JN378412 |
| Octaviania lineage A-2 | Japan, Nara Pref., Nara-shi, near Mt Kasuga | KPM-NC-0018020 | JQ619166 | – | – |
| Octaviania lineage A-3 | Japan, Tottori Pref., Hie Shirine | KPM-NC-0017770 | JN257994 | JN378470 | JN378413 |
| Octaviania lineage A-3 | Japan, Kyoto Pref., Nanzen-ji Shrine | KPM-NC-0017771 | JN257995 | JN378471 | JN378414 |
| Octaviania lineage A-3 | Japan, Kyoto Pref., Kyoto-gyoen | KPM-NC-0017773 | JN257996 | JN378472 | JN378415 |
| Octaviania lineage A-3 | Japan, Ehime Pref., Futami-cho | KPM-NC0017724 | JQ619167 | JQ619187 | – |
| Octaviania lineage A-4 | Japan, Nara Pref., Nara-shi, Nara Park | KPM-NC-0017776 | JN257997 | JN378473 | JN378416 |
| Octaviania lineage A-4 | Japan, Nara Pref., Nara-shi, Nara Park | KPM-NC-0018026 | JQ619169 | – | – |
| Octaviania lineage A-4 | Japan, Nara Pref., Nara-shi, near Mt Kasuga | KPM-NC-0018021 | JQ619168 | JQ619190 | JQ619184 |
| Octaviania lineage B | Japan, Hyogo Pref., Akou-shi | KPM-NC-0017778 | – | JN378474* | – |
| Octaviania lineage B | Japan, Kyoto Pref., Kyoto Botanical Garden | KPM-NC-0017780 | – | JN378475 | JN378417 |
| Octaviania lineage B | Japan, Okayama Pref., Mt Minagasen | KPM-NC-0017782 | – | JN378476 | JN378418 |
| Octaviania lineage B | Japan, Kyoto Pref., Uji-shi | KPM-NC-0017783 | JQ619171 | JN378477 | JN378419 |
| Octaviania lineage B | Japan, Nara Pref., Nara-shi, Mt Kasuga | KPM-NC-0017785 | JQ619170 | JN378478 | JN378420 |
| Octaviania lineage C | Japan, Tokyo, Hachioji-shi | KPM-NC-0017792 | – | JN378479 | JN378421 |
| Octaviania lineage C | Japan, Kanagawa Pref., Zushi-shi | KPM-NC-0017793 | JQ619173 | JN378480 | JN378422 |
| Octaviania lineage C | Japan, Tottori Pref., Tottori-shi, Ouchidani | KPM-NC-0017795 | – | JN378481 | JN378423 |
| Octaviania lineage C | Japan, Ehime Pref., Hutami-cho | KPM-NC-0017727 | JQ619172 | JQ619189 | – |
| Octaviania lineage D | Japan, Tottori Pref., Mt Daisen, Kagamiganaru | KPM-NC-0017798 | – | JN378482 | JN378424 |
| Octaviania lineage D | Japan, Akita Pref., near Lake Towada | KPM-NC-0017797 | JQ619174 | JN378483 | JN378425 |
| Octaviania lineage D | Japan, Tottori Pref., Mt Daisen | KPM-NC-0017806 | – | JN378484 | JN378426 |
| Octaviania lineage D | Japan, Tottori Pref., Yazu-cho | KPM-NC-0017810 | JQ619175 | JN378485 | JN378427 |
| Octaviania lineage D | Japan, Okayama Pref., Kagamino-cho | KPM-NC-0017812 | – | JN378486 | JN378428 |
| Octaviania lineage E | Japan, Kagoshima Pref., Amami-ohshima Isl. | KPM-NC-0017813 | JQ619176 | JN378487 | JN378429 |
| Octaviania lineage F | Japan, Miyagi Pref., shichigasyuku-cho | KPM-NC-0017814 | – | JN378488 | – |
| Octaviania lineage F | Japan, Kanagawa Pref., Minami-ashigara-shi | KPM-NC-0017829 | JQ619177 | JQ619188 | – |
| Octaviania lineage G | Japan, Hokkaido, Kamikawa-cho, Mt Daisetsu | KPM-NC-0017824 | JQ619178 | JN378489 | JN378430 |
| Octaviania lineage G | Japan, Hokkaido, Kamikawa-cho, Mt Daisetsu | KPM-NC-0017823 | – | JQ619185 | – |
| Octaviania lineage H | Japan, Okinawa Pref., Ishigaki Isl., Mt Omoto | KPM-NC-0017818 | JQ619179 | JN378490 | JN378431 |
| Octaviania lineage H | Japan, Okinawa Pref., Ishigaki Isl., Mt Omoto | KPM-NC-0017819 | JQ619180 | JN378491 | JN378432 |
| Octaviania lineage I | Japan, Toyama Pref., Nakashingawa-gun, Teteyama-cho | KPM-NC-0017822 | JQ619182 | JN378492 | JN378433 |
| Octaviania lineage I | Japan, Hyogo Pref., Kobe-shi, Kita-ku | KPM-NC-0017849 | JQ619183 | – | – |
| Octaviania lineage I | Japan, Kyoto Pref., Sonobe-cho | KPM-NC-0017820 | JQ619181 | – | – |
| Octaviania tasmanica | Australia, Victoria, East Gippsland, Alpine National Park, Black Mountain Road, Rams Horn track | Trappe 18104 | – | JN378493 | JN378434 |
| Octaviania tasmanica | Australia, Tasmania, Avre River Picnic Area, on road to Terhune Airwalk | OSC132097 | – | JN378494 | JN378435 |
| Octaviania tasmanica | Australia, Tasmania, Mount Field, Mt Field Nat. Park | MEL2341996 | – | JN378495 | JN378436 |
| Octaviania tasmanica | Australia, NSW, Southern Tablelands, off | MEL2128484 | – | JN378496 | JN378437 |
| Nungatta Rd., 3.5 km from junction with Imlay Rd, off small track to east | |||||
| Octaviania asterosperma | Spain, Sella Covallera | Trappe 23377 | JN257998 | JN378497 | – |
| Octaviania cyanescens | OR, Lane Co., Lanb Butte south of English Mountain | PNW FUNGI 5603 | – | JN378502 | JN378438 |
| Octaviania cyanescens | Canada, British Columbia | OSC58498 | – | JN378503 | JN378439 |
| Octaviania nigrescens sensu Singer & A.H. Sm. | USA, Maine, Tunk Lake, off route 182 | MES270 | – | JN378498 | JN378440 |
| Octaviania sp. | USA, Florida, Wakulla Co., Skipper Bay road, St Marks NW refuge | OSC131925 | – | JN378499 | JN378441 |
| Octaviania sp. (labeled as ‘O. asterosperma ’) | USA, Iowa, Story County, YMCA woods, Ames | KPM-NC-0017827 (RH30) | – | JN378500 | JN378442 |
| Octaviania sp. (labeled as O. asterosperma ‘ ’) | USA, Minnesota, Forestville State Park. | KPM-NC-0017828 (RH1181) | – | JN378501 | JN378443 |
| Rosbeevera eucyanea | Japan, Mie Pref., Kameyama-shi | TMI-40253 | – | HQ693879** | JN378444 |
| Rossbeevera westraliensis | Australia, Western Australia, Beedelup National Park, Anzac Rd | OSC61480 | – | JN378505 | JN378445 |
| Rossbeevera vittatispora | Australia, New South Wales, Genoa National Park, junction Imlay road and Nungatta road | OSC61484 | – | JN378506 | JN378446 |
| Rossbeevera vittatispora | Australia, New South Wales, c. 5 km south of the junction between Princess highway and Eden road | TO-AUS-46 | – | JN378507 | JN378447 |
| Leccinum aff. duriusculum | Japan, Hyogo Pref., Uwano | KPM-NC-0017830 | – | JN378510 | JN378448 |
| Leccinellum aff. griseum | Japan, Hyogo Pref., Uwano | KPM-NC-0017831 | – | JN378508 | JN378449 |
| Leccinellum aff. griseum | Japan, Tottori Pref., Tottori-shi, Ouchidani | KPM-NC-0017832 | – | JN378509 | JN378450 |
| Leccinum sp. (sect. Leccinum) | Japan, Iwate Pref., Appi | KPM-NC-0017838 | – | JN378512 | JN378452 |
| Leccinum sp. (sect. Leccinum) | Japan, Iwate Pref., Appi | KPM-NC-0017839 | – | JN378513 | JN378453 |
| Leccinum versipelle | UK, Scotland, Aberdeenshire, Dennet Oakwood National Nature Reserve | KPM-NC-0017833 | – | JN378514 | JN378454 |
| Leccinum scabrum | Japan, Iwate Pref., Appi | KPM-NC-0017837 | – | JN378511 | JN378451 |
| Leccinum scabrum | UK, Scotland, Aberdeenshire, Burn O’Vat | KPM-NC-0017840 | – | JN378515 | JN378455 |
| Leccinum vulpinum | UK, Scotland, Aberdeenshire, near Mar Lodge Estate | KPM-NC-0017834 | – | JN378516 | JN378456 |
| Tylopillus chlomapes | Japan, Tottori Pref., Yazu-cho | KPM-NC-0017835 | – | JN378517 | JN378457 |
| Austroboletus subvirens | Japan, Tottori Pref., Tottori-shi | KPM-NC-0017836 | – | JN378518 | JN378458 |
Table 2.
DNA sequences retrieved from GenBank.
| Taxon | Locality | Voucher No. | GenBank No. |
|
|---|---|---|---|---|
| ITS | nLSU | |||
| Octaviania sp. (= Octaviania lineage A-2) | Japan, Aichi Pref., Okazaki-shi | Orihara 227 | EU414289 | EU414290 |
| Octaviania sp. (= Octaviania lineage A-2) | Japan, Nara Pref., Nara-shi, Mt Kasuga | Orihara 231 | EU414291 | EU414292 |
| Octaviania asterosperma | UK, South Hampshire | RBG Kew K(M) 102511 | EU784378 | – |
| Octaviania asterosperma | UK, Caernarvonshire | RBG Kew K(M) 81259 | EU784379 | – |
| Octaviania asterosperma | Germany: Bavaria | Strain: Octa 1 | – | DQ534619 |
| Chamonixia caespitosa | USA | OSC 117571 | – | EU669260 |
| Chamonixia caespitosa | USA | Trappe 10517 | – | EU669427 |
| Chamonixia caespitosa | Germany | 92/83 | – | AF336245 |
| Leccinellum carpini (syn.: Leccinum pseudoscabrum) | Germany | 930808 | – | AF139691 |
| Leccinellum carpini (syn.: Leccinum pseudoscabrum) | Netherlands, Breukelen | hdb065 | – | AF454588 |
| Rossbeevera eucyanea | Japan, Mie Pref., Kameyama-shi | TMI-40253 | – | HQ693879 |
| Austroboletus mucosus | N/A | TH6300 | – | AY612798 |
| Austroboletus gracilis | USA, MA | Strain: 112/96 | – | DQ534624 |
| Austroboletus niveus | New Zealand | M / Strain: 312 | – | DQ534622 |
| Austroboletus novae-zelandiae | New Zealand | PDD7252 | – | DQ534623 |
Bayesian analyses were conducted with MrBayes 3.1.2 (Ronquist & Huelsenbeck 2003). For the nLSU dataset, the general time reversible model under the assumption of a discrete gamma-shaped rate variation with a proportion of invariable sites (GTR+I+G) was estimated as the best-fit likelihood model with MrModeltest 2.3 (Nylander 2004). The EF-1α dataset was further partitioned by codons and introns, and the best-fit likelihood models were estimated for each partition. For the 1st, 2nd, and 3rd codons and introns, HKY+I+G, F81+I, GTR+G, and GTR+G models were applied for the phylogenetic inference, respectively. For the combined analyses, the incongruence-length difference (ILD) test was conducted on PAUP* 4.0b10 beforehand to test compatibility of phylogenetic signals between the two concatenated DNA regions (Farris et al. 1995). We subsequently compared resultant tree topologies directly to confirm the compatibility. Posterior probabilities (PP) were approximated by the Metropolis-coupled Markov chain Monte Carlo method (Geyer 1991). Two parallel runs were conducted with one cold and seven heated chains each for 5 000 000 generations, starting with a random tree. Temperature of the seven heated chains was set to 0.15. Trees were saved to a file every 100th generation. We judged that the two runs reached convergence when the average SD of split frequencies continuously dropped below 0.01. Trees obtained before reaching convergence were discarded as the burn-in, and the remaining trees were used to calculate a 50 % majority consensus topology and to determine PP values for individual branches.
The nLSU and combined nLSU and EF-1α datasets were further analysed by the maximum likelihood (ML) method with RAxML v. 7.2.6 (Stamatakis 2006). The best-fit ML tree was inferred under the GTR+I+G model. The combined dataset was partitioned in the same way as in the Bayesian analysis, and different α-shape parameters, GTR rates, and empirical base frequencies were assigned to each partition. To check statistical support for the resultant tree topology, the rapid bootstrap option was used under the automatically assigned, GTA+CAT model, setting the number of replicates to 1 000.
The phylogenetic network analysis of the ITS dataset of one Octaviania lineage (lineage A) was conducted with SplitsTree 4 (Huson & Bryant 2006). Networks were constructed by the NeighborNet method under the setting of distance estimation to uncorrected P value and of ambiguous states of nucleotides as average states. The resultant networks were visualized with the ClusterNetwork method (Huson & Rupp 2008). Three O. asterosperma sequences (EU784378–EU784379 and JN257998) were used as outgroups.
RESULTS
Phylogenetic analyses of the nLSU dataset
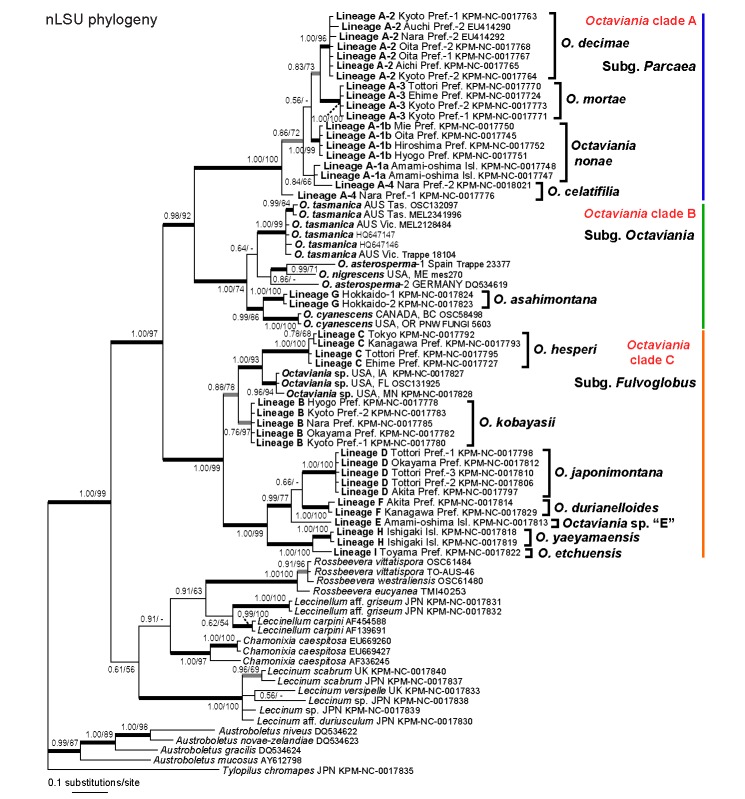
The final aligned nLSU dataset was 954 bp long. In the Bayesian inference, the two parallel MCMC runs converged after c. 930 000 generations. Accordingly, the first 9 300 trees in each run were discarded as the burn-in and the remaining 81 402 trees (representing c. 4.07M generations) were used to calculate a 50 % majority consensus tree and to determine PPs (Fig. 1). Likelihoods (ln L) of the best states for cold chains of the two runs were −4839.62 and −4863.13. Maximum likelihood analysis of the same dataset generated one ML tree (ln L = −4650.068186). Branches that were strongly supported by Bayesian PPs (> 0.95) were also present in the best ML topology. The resulting topology represented 10 infrageneric Japanese lineages that were also represented in the multi-gene analyses (i.e., lineages A-2–A-3 and B–I). The phylogeny recovered the three clades within Octaviania (clade A–C). Unfortunately only part of the nLSU sequences (557 bp in total) were successfully obtained in one specimen of the lineage A-4 (KPM-NC0017776). Although it was 99 % identical to another sequence of the lineage A-4 (KPM-NC0018021), the monophyly of the lineage was collapsed and the latter sequence formed a clade with the lineage A-1a with weak statistic supports (PP = 0.84, BS = 66 %). Accordingly, monophyly of the lineages A-1a and A-1b was not recovered in the nLSU analyses. Monophyly of Octaviania lineage B sequences was not supported in the Bayesian analysis despite that all the sequences were identical.
Fig. 1.
Bayesian 50 % majority-rule consensus tree of the nLSU rDNA dataset of Octaviania and allied genera. Bayesian posterior probabilities (PP) and RAxML bootstrap (BS) values (1 000 replicates; only BS > 50 % are shown) are indicated above or below branches or at nodes as PP/BS. Branches supported by PP greater than 0.95 and BS greater than 70 % are depicted as black thickened lines. Branches supported by either PP less than 0.95 or BS less than 70 % are depicted as grey thickened lines.
Phylogenetic analyses of the EF-1α dataset
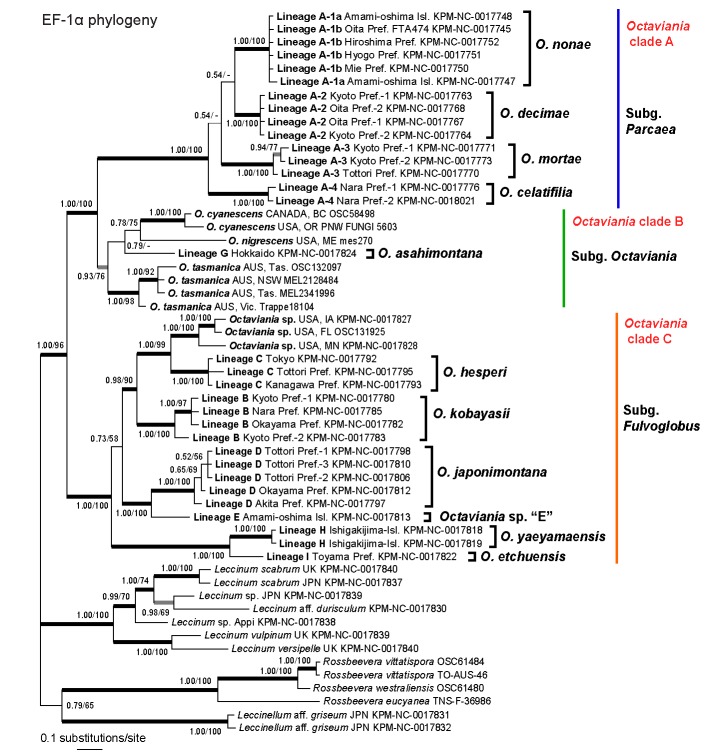
Preliminary analyses that included either Tylopilus chromapes (JN378457) or Austroboletus subvirens (JN378458) as an outgroup produced paraphyly of the Octaviania clade. This is likely due to long branch attraction caused by the highly divergent T. chromapes and A. subvirens sequences so we excluded them and re-analysed a dataset without an outgroup. The final EF-1α dataset was 1 145 bp long and included 55 taxa. In the Bayesian inference, the two MCMC runs converged after c. 590 000 generations. Accordingly, the first 5 900 trees in each run were discarded as the burn-in and the remaining 88 202 trees (representing c. 4.41M generations) were used to calculate a 50 % majority consensus tree and determine PPs (Fig. 1). Likelihoods (ln L) of the best states for cold chains of the two runs were −4370.99 and −4378.93. The final optimization likelihood score was ln L = −4308.079165. Branches that were strongly supported by Bayesian PPs (> 0.95) were also present in the best ML topology.
Bayesian and ML analyses based on EF-1α detected 11 species-level lineages (lineages A-1–A-4, B–E and G–I) among the Japanese collections. EF-1α sequences of Octaviania lineage F were not available so that these species were not included in the analyses. Lineages A-1a and A-1b were not differentiated in the EF-1α phylogeny and these two groups formed instead a monophyletic clade with strong statistic supports (PP = 1.00, BS = 100 %). As in the nLSU phylogeny, Octaviania spp. were divided into three clades (A–C) although monophyly of clade B was not strongly supported by the Bayesian analysis (PP = 0.93).
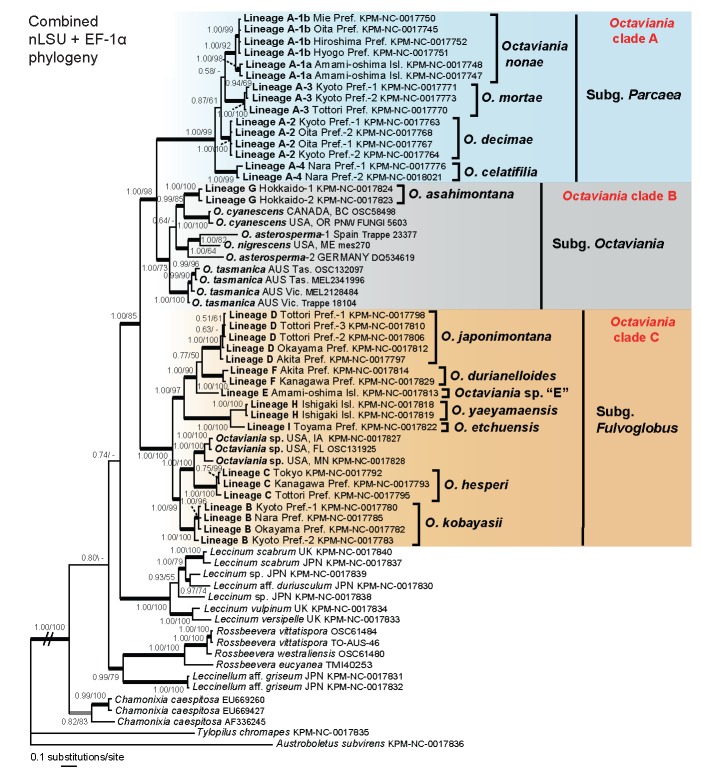
Phylogenetic analyses of the combined nLSU and EF-1α dataset
The ILD test found no significant incongruence between the nLSU and EF-1α datasets. There was also no topological incongruence in significantly supported clades between the nLSU and EF-1α phylogenies (highlighted with thicked black lines in Fig. 1, 2). Thus, they were combined into a nucleotide alignment that was 2105 bp long. In the Bayesian inference, the two parallel MCMC runs reached convergence after c. 780 000 generations so the first 7 800 trees were discarded as the burn-in. The remaining 84 402 trees (representing c. 4.22M generations) were used to calculate a 50 % majority consensus tree and determine PPs (Fig. 3). Likelihoods (ln L) of the best states for cold chains of the two runs were −9878.35 and −9886.34. Maximum likelihood analysis of the multigene dataset resulted in one ML tree (ln L = −9787.186452). Branches that were strongly supported by Bayesian PPs (> 0.95) were also present in the best ML topology.
Fig. 2.
Bayesian 50 % majority-rule consensus tree of the EF-1α dataset of Octaviania and allied genera. Bayesian posterior probabilities (PP) and RAxML bootstrap (BS) values (1 000 replicates; only BS > 50 % are shown) are indicated above or below branches or at nodes as PP/BS. Branches supported by PP greater than 0.95 and BS greater than 70 % are depicted as black thickened lines. Branches supported by either PP less than 0.95 or BS less than 70 % are depicted as grey thickened lines.
Fig. 3.
Bayesian 50 % majority-rule consensus tree of the combined nLSU rDNA and EF-1α (tef1) dataset of Octaviania and allied genera. Sequences of Austroboletus subvirens and Tylopilus chlomapes were used for outgroups. Bayesian posterior probabilities (PP) and RAxML bootstrap (BS) values (1 000 replicates; only BS > 50 % are shown) are indicated above or below branches or at nodes as PP/BS. Branches supported by PP greater than 0.95 and BS greater than 70 % are depicted as black thickened lines. Branches supported by either PP less than 0.95 or BS less than 70 % are highlighted as grey thickened lines.
Both Bayesian and ML multigene analyses supported monophyly of Octaviania and represented 12 phylogenetically divergent infrageneric lineages from Japan (lineages A-1, A-2–A-4 and B–I) that were different from all of the foreign taxa included in the analyses (Fig. 3). The genus Octaviania was divided into three phylogenetically distinct clades (clade A–C). Clade A is comprised of four genetically distinct but morphologically and ecologically similar cryptic species (i.e., lineages A-1, A-2, A-3, and A-4). The lineage A-1 diverged further into two lineages that were geographically isolated by a strait with strong statistical supports (i.e., lineages A-1a and A-1b). The relationships among these four lineages, however, were not resolved in both Bayesian and ML analyses. Clade B contains sequences of the type species, O. asterosperma, from Germany and Spain (Binder & Hibbett 2006 and this study) as well as several North American and Australian species (i.e., O. cyanescens Trappe & Castellano, O. nigrescens (Zeller) Singer & A.H. Sm. and O. tasmanica (Kalchbr. ex Massee) Lloyd). The two sequences identified as O. asterosperma (i.e., DQ534619 and JN378497) actually represent two different species. The only Japanese collection placed within clade B was lineage G, collected on the island of Hokkaido in northern Japan. Clade C contains the other Japanese lineages and one unidentified North American species. Although the multi-gene analyses produced a well-resolved phylogeny of the genus Octaviania, the relationship between Octaviania and its sister group remained unresolved.
ITS sequences of Japanese Octaviania lineages
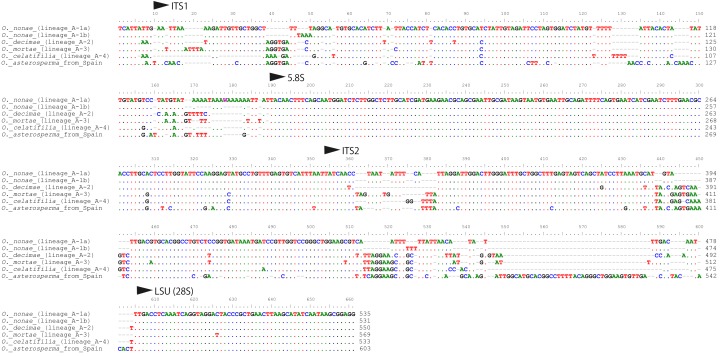
The ITS nuc-rDNA sequences of the lineages A1–A4 were obtained successfully in most cases (Table 1). Those ITS1-5.8S-ITS2 regions were 468–506 bp long. On the other hand, PCR amplicons of the ITS of the lineages B–I are often unusually long and showed polymorphism within individual specimens. Thus, only limited numbers of ITS sequences were obtained successfully. The total length of their ITS1-5.8S-ITS2 region was 709–1461 bp. The sequences had a long insertion within the ITS2 region that will be analysed and discussed in detail in a subsequent publication. It was difficult to align these insertions because they had considerable numbers of gaps and substitutions. Nevertheless, the ITS sequences excluding the insertion had 98 % similarity within each lineage.
Morphological comparison and ITS network analysis of cryptic species in Clade A
The four cryptic lineages A-1–A-4, which were strongly supported in the EF-1α and the combined analyses (Fig. 2, 3), were further examined based on morphology and ITS phylogenetic analyses. We found that the combination of basidiospore size and spore ornamentation were diagnostic to distinguish lineages A-1, A-2, and A-3 (Table 3, Fig. 7d, 8c, 9c). However, there was no statistically significant difference in both the characters between the lineages A-2 and A-4 (P = 0.05, based on ANOVA, data not shown). Basidia of the Octaviania lineage A-3 were significantly shorter than those of the other lineages, suggesting that this character was also useful for species delimitation. There were no clear morphological differences between lineages A-1a and A-1b and analysis of ITS recovered a single monophyletic group. This result was in contrast to the nLSU analysis but congruent with results based on both the EF-1α and multi-gene analyses.
Table 3.
Comparison of micro- and macroscopic characters among four cryptic species in the Octaviania lineage A. Average dimensions (n = 50) are described in bold.
| Octaviania nonae (= lineage A-1) | Octaviania decimae (= lineage A-2) | Octaviania mortae (= lineage A-3) | Octaviania celatifilia (= lineage A-4) | |
|---|---|---|---|---|
| Size of basidiospores | 8.5–(8.9−)10.9–13.3(−13.8) × | (10−)10.3–11.8(−13.4) × | 11.1–(11.3−)13–14.9(−15.6) × | (9.9−)11.4–12.9(−13.2) × |
| (excluding spines; μm) | (8−)8.1–9.8–11.6(−12.4) | (9.3−)10.8–12.4(−12.9) | 10.2–(10.4−)12.1–14.1(−15.6) | (9.3−)9.4–10.8(−12)–12.1 |
| Size of spiny ornaments | 1.5–(1.6−)2.2–2.9 × | 1.6–(1.7−)2.7–3.7(−4.2) × | 1.9–2.8(−3.6)–3.7 × | 1.6–(1.8−)2.6–3.6 × |
| (length × width; μm) | 1.1–(1.5−)2.5–3.9(−4.4) | 1.7–(1.9−)3.1(−4.4)–4.5 | 1.9–(2−)3.2(−4.2)–4.5 | 1.7–2.9(−4)–4.1 |
| Characteristics | Small basidiospores, small ornamentation and clavate basidia | Medium-sized basidiospores, large ornamentation and clavate basidia | Large basidiospores, large ornamentation and short clavate basidia | Medium-sized basidiospores, large ornamentation and clavate basidia; fruitbody often stained with pale yellow |
Fig. 7.

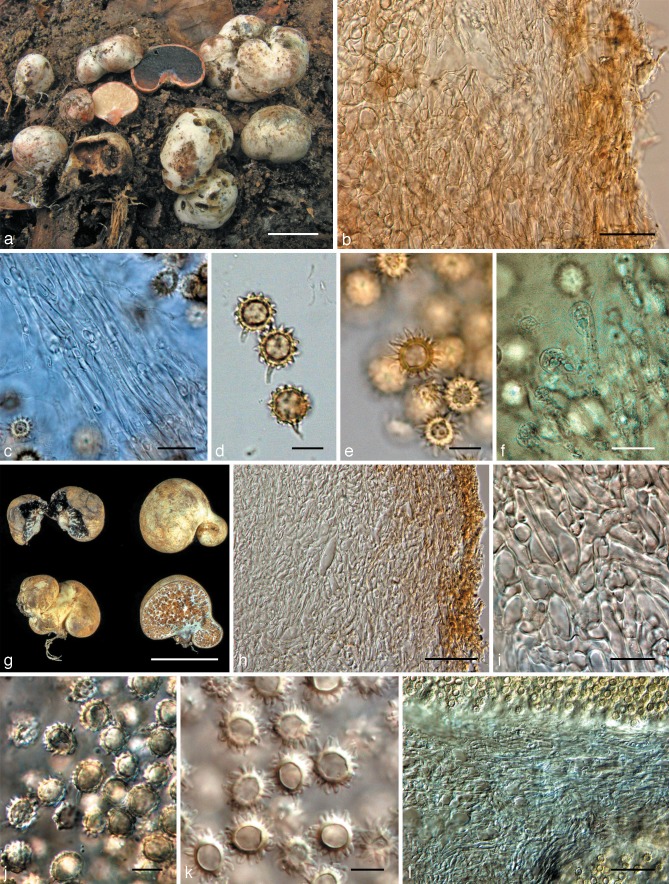
Octaviania nonae. a. Basidiomata (holotype); b. peridium (upper: peridiopellis; lower: context; KPM-NC0017751); c. basidiospores mounted in water (holotype); d. SEM image of basidiospore (holotype); e. basidium mounted in lactoglycerol and observed under DIC microscope (holotype); f. ectomycorrhizas (arrow; holotype). — Scale bars: a = 1 cm; b = 30 μm; c, e = 10 μm; d = 5 μm; f = 1 mm.
Fig. 8.
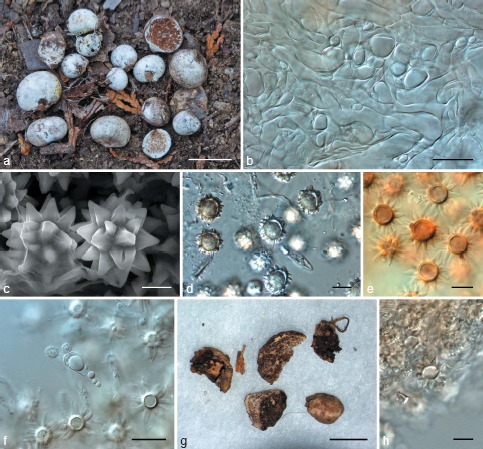
a–f: Octaviania decimae. a. Basidiomata (KPM-NC0017768); b. context of peridium (KPM-NC0017768); c. SEM image of basidiospore (holotype); d. basidiospores mounted in water (KPM-NC0017768); e. basidiospores mounted in LCB after presoaking with Melzer’s reagent (KPM-NC0017762); f. 2-spored basidium (KPM-NC0017768). — g, h: “O. tuberculata” sensu Yoshimi & Doi (TNS-F-183206). g. Basidiomata; h. basidiospore mounted by lactoglycerol after presoaking with 3 % KOH. — Scale bars: a = 1 cm; b, f = 20 μm; c = 5 μm; d, e, h = 10 μm; g = 5 mm.
Fig. 9.
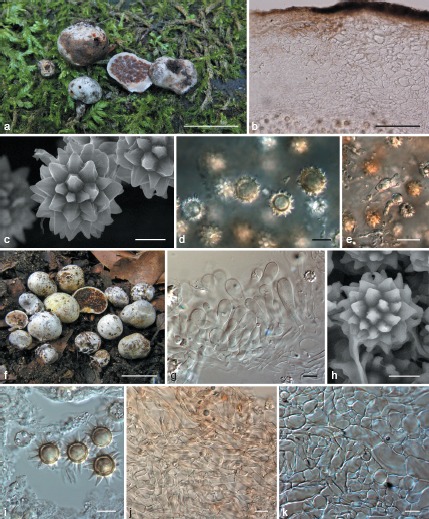
a–e: Octaviania mortae. a. Basidiomata (Orihara 1208); b. peridium (KPM-NC0017770); c. SEM image of basidiospores (holotype); d. basidiospores mounted in water (KPM-NC0017772); e. basidium and basidiole mounted in water (KPM-NC0017772). — f–k: Octaviania celatifilia (holotype). f. Basidiomata; g. undeveloped hymenium; h. SEM image of basidiospore; i. basidiospores mounted by 3 % KOH; j. peridiopellis; k. context of peridium. — Scale bars: a, f = 1 cm; b = 100 μm; c, h = 5 μm; d, g, i, k = 10 μm; e = 20 μm.
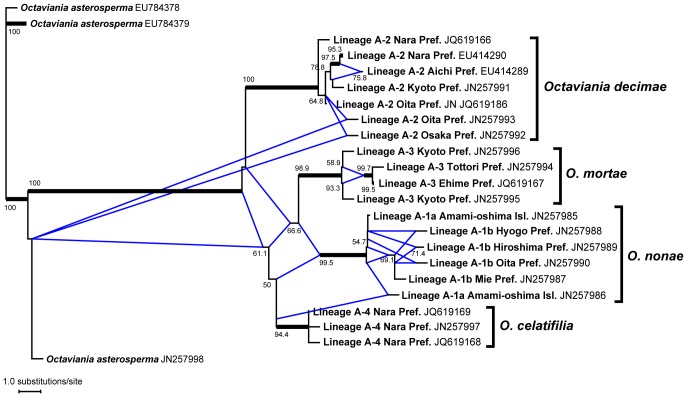
The finally aligned dataset of partial SSU-ITS1-5.8S-ITS2-partial LSU sequences was 735 bp long. To infer not only precise phylogenetic relationships but also traces of recent hybridization among these lineages, we carried out the phylogenetic network analysis using the NeighborNet method. The ITS network (Fig. 4) did not suggest any hybridization among the four extant lineages (A-1–A-4) despite the fact that they are sympatric and occur in the same forest habitat. The four lineages are 88–94 % similar across the ITS1-5.8-ITS2 region in the NCBI BLAST search (Query coverage = 88–100 %; e-value < 1; Fig. 5).
Fig. 4.
Phylogram of the cluster network based on the nuclear ITS rDNA dataset of Octaviania lineage A1–A4 constructed by the NeighborNet method. Octaviania asterosperma sequences (EU784378–784379, JN257998) were used to root the tree. Bootstrap values greater than 50 % are indicated at edges (1 000 replicates). Edges supported by bootstrap values greater than 95 % are highlighted as black thickened lines. Reticulate edges shown as blue lines indicate possible two pathways leading to the descendant lineages in the phylogeny, leaving a possibility of hybridization between lineages.
Fig. 5.
Comparison of ITS1-5.8S-ITS2 sequences of the Octaviania lineages A1a–b and A2–A4. Voucher numbers of the sequences used for the comparison are as follows: Octaviania lineage A-1a: JN25986; lineage A-1b: JN257990; lineage A-2: JN257991; lineage A-3: JN257994; lineage A-4: JN257997; O. asterosperma: JN257998.
Ultrastructure of basidiospore ornaments
TEM observation of mature basidiospores of Japanese Octaviania showed variation in both the number and shape of cavities inside basidiospore ornaments among the different lineages (Fig. 6, and see individual species descriptions). Although not always distinctive, these cavities were also visible with light microscopy at ×1 000. Under the light microscope, these cavities often look like striations on the surface of spore ornaments, but the TEM photomicrographs showed that they were actually isolated from the surface structure in most cases (Fig. 6b, d). All of the specimens from clade A that we observed had multiple cavities inside their spore ornaments, whereas representatives of some lineages within clades B and C (e.g., lineages D, E, H, and I) had only a single, simple cavity.
Fig. 6.
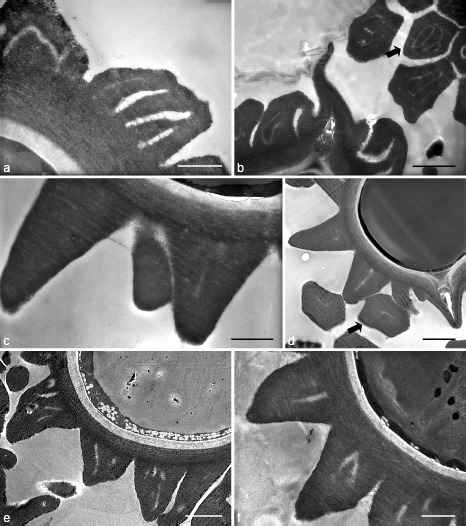
SEM image of basidiospore ornaments of Octaviania spp. a, b. O. hesperi (lineage C; KPM-NC0017789): a. vertical section; b. vertical section of the bottom part of the basidiospore and horizontal sections of the ornaments (arrow). — c, d. O. japonimontana (lineage D; KPM-NC0017798, holotype). c. Vertical section; d. vertical section of a part of the basidiospore and horizontal sections of the ornaments (arrow). — e. O. decimae (lineage A-2; KPM-NC0017757). — f. O. durianelloides (lineage F; KPM-NC0017815). — Scale bars: a, c, e, f = 1 μm; b, d = 2 μm.
TAXONOMY
Octaviania Vittad., Monogr. Tuberac.: 15. 1831, emend. Orihara
Typus generis. Octaviania asterosperma Vittad., Monogr. Tuberac.: 17. 1831.
Basidiomata solitary to gregarious, ectomycorrhizal, hypogeous to emergent, mostly less than 5 cm diam, globose, subglobose, reniform or tuberiform, more or less rubbery, sessile or with a rudimentary stipe at the base, surface glabrous, floccose, or occasionally scaly to warty, often discolouring when rubbed or bruised. Peridium persistent, context often discolouring when exposed to air, composed of often inflated, more or less interwoven, filamentous hyphae often intermingled with isodiametric to spherical cells especially at maturity and pigmented to colourless, filamentous, granular hyphae; true sphaerocysts absent. Gleba whitish at first, becoming brown to blackish at maturity, composed of variously sized, subspherical to hemispherical chambers up to c. 2.5 mm diam, often filled with reddish brown to brown to blackish brown spore mass; each glebal chamber permeated by veins of glebal trama almost concolorous with peridial context to form a marbled gleba. Sterile base usually absent to pulvinate, occasionally more or less dendroid, occasionally discolouring when exposed to air, of dense, interwoven, sometimes inflated, filamentous hyphae. Cystidia absent. Hymenium present but poorly developed in most species, with basidia and interspersed, clavulate to cylindro-clavate basidioles. Subhymenium absent or if present then poorly developed. Basidia clavulate to cylindro-clavate or doliiform, hyaline, 2–4-spored. Basidiospores globose to ellipsoid, thick-walled, more or less dextrinoid, non-amyloid, covered with large, thick-walled, pyramidal to conical ornaments that are polygonal at the base but in some species several spore ornaments adhering to form irregular ridges, with one to several cavities inside ornaments; ornaments usually expand and elongate when soaked in alkaline or acidic solutions; perisporium and ectosporium absent or indistinct. Clamp connections absent in all tissues.
Octaviania subgenus Parcaea Orihara, subg. nov. — MycoBank MB563165
Typus subgeneris. Octaviania nonae Orihara.
Etymology. The Latin, Parcae, is the name of the three goddesses of fate in Roman mythology (= the Fates), metaphorically expressing that the subgenus contains three morphologically similar sister species named after these three goddesses (i.e., Nona, Decima, and Morta).
Basidiomata solitary to gregarious, hypogeous to emergent, mostly up to 2.5 cm diam, subglobose, depressed-globose, tuberiform or reniform, often with white to whitish rhizomorphs at the basal rudimentary stipe, surface glabrous, white to dirty white occasionally with yellowish to dirty yellow tints, often gradually turning dark grey to black when touched, rubbed or bruised. Peridium 0.3–2 mm thick, as the interior concolorous with the surface, hyphae of context filamentous when immature, becoming inflated and thick-walled at maturity. Gleba wet, whitish to pale brown when immature, becoming strongly glutinous, fuscous or chocolate-brown to blackish brown at maturity. Hymenium and subhymenium indistinct, deliquescent at maturity. Basidia clavate to clavulate. Basidiospores spiny, pale cinnamon to brown, strongly dextrinoid, with pyramidal ornaments that each contain one to several slit-like cavities inside.
Notes — Octaviania subg. Parcaea is newly proposed to accommodate species belonging to clade A (i.e., O. nonae, O. decimae, O. mortae, and O. celatifilia). The subgenus is morphologically characterized by the presence of a thick, white to whitish peridium that gradually discolours to almost black when rubbed or bruised, and the lack of a distinct hymenial layer (i.e., basidia and basidioles are sparsely embedded in a glutinous spore mass). Ecologically, the strong host preference for evergreen plants in the family Fagaceae (e.g., Quercus glauca Thunb. and Castanopsis spp.) is a common feature, suggesting this group is probably of temperate or subtropical origin.
Of all the known species of Octaviania, O. lanigera Hesse is most morphologically similar to the members of subg. Parcaea in having a remarkably thick peridium (c. 1 mm thick) as well as other macroscopic similarities. We have morphologically examined an authentic specimen of O. lanigera collected by R. Hesse in Germany in September 1901 (No. NY780603). Basidiospores of O. lanigera were more yellowish than those of the species in subg. Parcaea described below, and O. lanigera spores also had larger spiny ornaments with multiple, distinct cavities inside (2.1–4.6 μm (mean 3.4 μm) wide, n = 30). These characteristics, as well as its association with Betula, imply the affinity of the species with O. asahimontana Orihara sp. nov. rather than the species in subg. Parcaea.
Octaviania nonae Orihara, sp. nov. — MycoBank MB563166; Fig. 7; Map 1
Map 1.
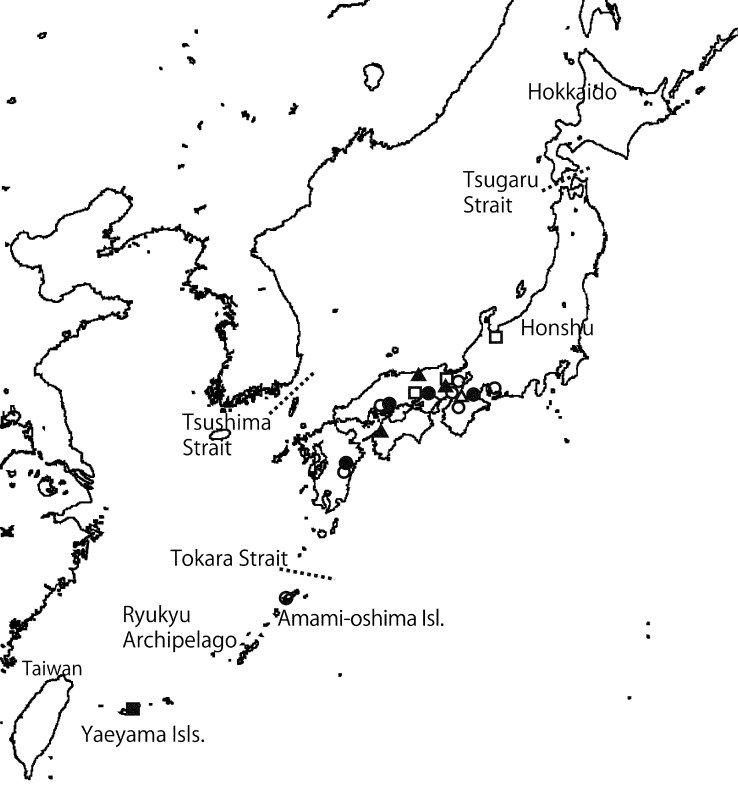
Geographic distribution of the species of subg. Parcaea (= Octaviania nonae (●), O. decimae (○), O. mortae (▲), O. celatifilia (▵)) and O. yaeyamaensis (■), O. etchuensis (□).
Etymology. Latin, Nona, is the name of one of the three goddesses of fate in Roman mythology. Nona was the first of the three deities and spun threads of human life, expressing metaphorically that O. nonae is a member of the cryptic species group.
Basidiomata solitary or in small clusters, up to 2.2 cm diam but mostly less than 1.5 cm diam, subglobose to depressed globose, firm, rubbery, sessile or with a concolorous, rudimentary stipe at the base, surface glabrous, white to greyish white, gradually becoming dark grey to black when touched, rubbed or bruised, sometimes with sparse mycelial tufts concolorous with the peridial surface, basal rhizomorphs sparse, white, narrow, easily snapping off from the base of basidiomata. Peridium 0.3–1.2 mm thick when fresh, 150–500 μm thick in dried specimens, white, context white to cream, not discoloured when cut. Gleba pale brownish when immature, becoming fuscous to blackish brown at maturity, composed of various-sized, subspherical to ellipsoidal chambers enclosed by white glebal tramae forming a marbled pattern, glebal chambers filled with a dense, glutinous matrix. Sterile base pulvinate, greyish white, often connected to a short, rudimentary stipe, gradually turning blackish when exposed to air. Ectomycorrhiza plurifurcate, glabrous, more or less sinuate, light-brown to pale reddish brown. Odour unpleasant, a mixture of sweet and dimethyl sulfide smells.
Basidiospores globose to subglobose or broadly ellipsoid, spinose, pale cinnamon, dextrinoid, 8.5–(8.9−)13.3(−13.8) × (8−)8.1–11.6(−12.4) μm, mean 10.9 × 9.8 μm (standard deviation [SD]: 1.18 [length], 0.88 [width]), Q = 1–1.32, walls 0.8–2.1 μm thick, spinose ornaments ([length] × [width at the base]) 1.5–(1.6−)2.9(−3) × 1.1–(1.5−)3.9(−4.5) μm, mean 2.2 × 2.5 μm, more or less angular at the base, internal cavities of ornaments slit-like in vertical section, labyrinthiform in horizontal section but sometimes indistinct. Basidia clavate, hyaline, 2–4-spored, 25–38.5 × 7.5–11 μm, sterigmata up to 9.5 μm long. Basidioles clavate, hyaline, almost the same size as basidia. Hymenia poorly developed, composed of basidia and interspersed basidioles. Subhymenium absent. Glebal trama of parallel, non-inflated, thin-walled (up to 1 μm thick), white, filamentous hyphae 2–5.5 μm broad. Sterile base of densely interwoven, more or less inflated, thin-walled (up to 1 μm thick), white to colourless, filamentous hyphae 3–12 μm broad; outermost hyphae mostly dark brown. Peridium 300–1200 μm thick (150–550 μm thick in dried specimens): peridiopellis up to 40 μm thick, dark brown, composed of straight, partly inflated, filamentous hyphae 2–12 μm broad forming a cutis, parallel to subparallel to surface, walls up to 1.5 μm thick; peridial context white, of interwoven, inflated, thin- or thick-walled (0.5–2 μm thick) filamentous hyphae 2.3–15 μm broad, hyphae in inner part narrower than in outer part, intermingled with scattered, isodiametric to subspherical cells up to 70 μm diam at maturity. Clamp connections absent in all tissues.
Habitat, Distribution & Season — Hypogeous or partially emergent under Castanopsis sieboldii (Makino) Hatus, C. cuspidata (Thunb. ex Murray) Schottky, and Quercus glauca; Honshu, Kyusyu, and Amami-oshima Isl. (Japan); autumn to early spring (October to March).
Holotype. Japan, Kagoshima Pref., Amami-oshima Isl., Yamato-son, north-eastern foot of Mt Yuwandake, under Castanopsis sieboldii subsp. lutchuensis, 29 Nov. 2008, T. Orihara, KPM-NC0017749 (Orihara 945; isotype TNS-F-41401).
Other specimens examined. Japan, Kagoshima Pref., Amami-oshima Isl., Oshima-gun Ukon-son, eastern foot of Mt Yuwandake, 22 Nov. 1988, Y. Doi, TNS-F-11480 (Yoshimi 7397) (labelled as ‘Octaviania nigrescens’); the same locality, 22 Nov. 1988, Y. Doi, TNS-F-11479 (Yoshimi 7401); Amami-oshima Isl., Yamato-son, north-eastern foot of Mt Yuwandake, under Castanopsis sieboldii subsp. lutchuensis, 17 Nov. 2007, T. Orihara, KPM-NC0017746 (Orihara 757); the same locality, 29 Nov. 2008, T. Orihara, KPM-NC0017748 (Orihara 944); Amami-oshima Isl., Tatsugoh-cho, En, under Castanopsis sieboldii subsp. lutchuensis, 19 Nov. 2007, T. Orihara, KPM-NC0017747 (Orihara766); Hiroshima Pref., Hiroshima-shi, Higashi-ku, Hiroshima Prefecture Ryokka-Center, under Q. glauca, 23 Oct. 2010, A. Hadano, KPM-NC0017752 (Orihara1290); Hyogo Pref., Kobe-shi, Shiogahara, under C. cuspidata, 21 Mar. 2010, M. Ohmae, KPM-NC0017751 (Orihara1157); the same locality, 21 Nov. 2010, T. Muroi, KPM-NC0017753 (Orihara1375); Mie Pref., Kameyama-shi, Seki-cho, under C. cuspidata, 31 Oct. 2009, H. Miwa, KPM-NC0017750 (Orihara1114); Oita Pref., Saiki-shi, Shiroyama, under C. sieboldii, 15 Jan. 2011, T. Orihara, KPM-NC0017745 (Orihara1362).
Notes — Thus far we have not found any macroscopic diagnostic character to separate O. nonae from other species in subg. Parcaea; microscopically the combination of its small basidiospores (mean diam 10.9 μm) and spore ornaments (mean height 2.2 μm) is unique to O. nonae. Although O. nonae co-occurs with its sister species O. decimae at some sites (e.g., the specimens collected from Hiroshima and Oita Prefectures; Map 1), they are nonetheless well-supported as distinct species in analyses of ITS, nLSU, and EF-1α (Fig. 1, 2, 3 and 4). Maximum identities of the ITS1-5.8S-ITS2 sequence with the other species of subg. Parcaea (i.e. O. decimae, O. mortae, and O. celatifilia) in the BLAST search were 88–91 % (Query coverage = 89–94 %).
The ITS and nLSU sequences of O. nonae specimens collected from Amami-oshima Island showed slight differences from the other sequences of the species collected from the main island (Honsyu) and Kyusyu (sequence similarity: 97.4 % in ITS and 99.1 % in nLSU [KPM-NC0017748 vs KPM-NC0017750]; e-value = 0; Query coverage = 99–100 %]). This might be due to ancient geological disjunction between the northern Ryukyu Archipelago and the mainland of Japan by the Tokara Strait, which demarcates the border between the Palearctic and Oriental faunal regions. However, it is difficult to examine reproductive ability of their F1 strain because mating tests are not practical. In addition, our analyses show phylogenetic continuity between the two lineages and we therefore currently consider them as a single species.
Octaviania decimae Orihara, sp. nov. — MycoBank MB563167; Fig. 6e, 8; Map 1
Etymology. Latin, Decima, is the name of one of the three Roman goddesses of fate, who measured the threads of human life, expressing metaphorically that the species is a member of the cryptic species group.
Basidiomata solitary or sparse, up to 2.3 cm diam but mostly less than 1.5 cm diam, subglobose to depressed globose to reniform, firm, rubbery, sessile or with concolorous, rudimentary stipe at the base, surface glabrous, white to greyish white, occasionally with whitish yellow to yellow-brown or more rarely bluish green patches, gradually becoming dark grey to black when touched, rubbed or bruised, rhizomorphs sparse, white, narrow, easily snapping off from the base of basidiomata. Peridium 0.3–1.2 mm thick when fresh, white, context white to cream colour, rarely turning immediately indigo when cut. Gleba pale brownish when immature, becoming dark brown to blackish brown at maturity, structure almost the same as O. nonae, trama rarely turning immediately indigo when cut. Sterile base pulvinate, greyish white, often connected to a short stipe, gradually turning blackish when exposed to air. Odour unpleasant, like a mixture of sweet and dimethyl sulfide smells.
Basidiospores globose to subglobose or broadly ellipsoid, spinose, dextrinoid, (10−)10.3–13.4 × 9.3–12.4(−12.9) μm, mean 11.8 × 10.8 μm (SD: 0.78 [length], 0.75 [width]), Q = 1–1.23, walls 1–2.3 μm thick, spinose ornaments 1.6–(1.7−) 3.7(−4.2) × 1.7–(1.9–4.4)–4.5 μm, mean 2.7 × 3.1 μm, pyramidal, internal cavities of ornaments slit-like in vertical section, labyrinthiform to zonate to cochleae in horizontal section. Basidia clavate, hyaline, 2–4-spored, 24–37 × 7.5–11.5 μm, mean 31.5 × 9.9 μm, sterigmata up to 11 μm long. Basidioles clavate, hyaline, almost the same size as basidia. Hymenia poorly developed, composed of basidia and interspersed basidioles. Subhymenium absent. Glebal trama of subparallel, straight or sinuate to strangulated, non-inflated, thin-walled (up to 0.5 μm thick), white, filamentous hyphae 2.2–11.4 μm broad. Sterile base of densely interwoven, partly inflated, white to colourless, filamentous hyphae 2–15 μm broad, walls 0.5–1.5 μm thick; in outermost part the hyphae mostly dark brown, narrower. Peridium 300–1200 μm thick (200–700 μm thick in dried specimens): peridiopellis up to 80 μm thick, colourless to dark brown, composed of repent, straight, non-inflated, thin-walled (up to 0.5 μm thick), filamentous hyphae 1.7–10 μm broad, subparallel to surface; peridial context white to colourless, of densely interwoven, inflated, thin- or thick-walled (0.5–1.5 μm thick) filamentous hyphae 2–17 μm broad, hyphae in inner part narrower than in outer part, intermingled with scattered, isodiametric to subspherical cells up to 25 μm diam at maturity. Clamp connections absent in all tissues.
No distinct morphological and ecological differences from O. celatifilia (= Octaviania lineage A-4) but c. 90 positions in nuclear ITS rDNA sequence are consistently different between the two species (Fig. 5).
Habitat, Distribution & Season — Hypogeous or partially emergent under Castanopsis sieboldii, C. cuspidata, and Quercus glauca; Honshu, Kyusyu (Japan); early summer to winter (June to January).
Holotype. Japan, Kyoto Pref., Kyoto-shi, Sakyo-ku, south-western foot of Mt Hiei, under Q. glauca, 5 July 2008, A. Kajiyama & T. Orihara, KPM-NC0017763 (Orihara 808; isotype TNS-F-41402).
Other specimens examined. Japan, Kyoto Pref., Kyoto-shi, Sakyo-ku, south-western foot of Mt Hiei, under Q. glauca, 17 July 2004, T. Orihara, KPM-NC0017754 (Orihara 128); the same locality, 28 June 2004, T. Orihara, KPM-NC0017756 (Orihara 216); the same locality, 10 June 2006, T. Orihara, KPM-NC0017760 (Orihara 394); the same locality, 3 Sept. 2007, T. Orihara, KPM-NC0017762 (Orihara 681); Kyoto-shi, Sakyo-ku, Mt Uryu, 13 June 2004, M Kutsuna, KPM-NC0017755 (Orihara 147); the same locality, 3 Nov. 2007, H. Sasaki, KPM-NC0017757 (Orihara 747); Kyoto-shi, Saikyo-ku, Mt Ponpon, in Q. glauca and Castanopis forest, T. Matsumiya, KPM-NC0017764 (Orihara 1121); Nara Pref., Nara-shi, Mt Kasuga, 11 Dec. 2004, T. Orihara, KPM-NC0015344 (Orihara 231); the same locality, 11 July 2005, T. Orihara, KPM-NC0017759 (Orihara 262); the same locality, 3 Nov. 2006, T. Orihara, KPM-NC0017761 (Orihara 536); the same locality, under Q. givla, 24 Dec. 2011, T. Orihara, KPM-NC0018020; Aichi Pref., Okazaki-shi, in Q. glauca and Q. serrata forest, 30 Nov. 2004, S. Honda, KPM-NC0015343 (Orihara 227); Okazaki-shi, Ohata-sho, Nishiyama, under Q. serrata, 7 Nov. 2009, S. Honda, KPM-NC0017765 (Orihara 1318) & TNS-F-41403 (duplicate); Hiroshima Pref., Hiroshima-shi, Higashi-ku, Fukuda, in litters under Q. serrata, Q. glauca, and P. densiflora, 18 Oct. 2009, T. Imoto, KPM-NC0017766 (Orihara 1354); Oita Pref., Saiki-shi, Ume-Oaza, Shigeoka, 1.4 km south-west of Sotaro Station, under C. sieboldii, 16 Jan. 2011, Y. Sunada & T. Orihara, KPM-NC0017767 (Orihara 1367); Oita-shi, Oaza, Hisado, Yayama, under Q. glauca, 17 Jan. 2011, A. Hadano & T. Orihara, KPM-NC0017768 (Orihara 1373) & TNS-F-41404 (duplicate).
Notes — This species, which is the most common among the species in subg. Parcaea, has intermediate microscopic features between O. nonae and O. mortae. The average basidiospore size of O. decimae is between those of O. nonae and O. mortae although the basidiospore ornaments are larger than those of O. nonae and similar to those of O. mortae (Table 3). Another slight difference between O. decimae and the other two species is that O. decimae sometimes has whitish yellow to yellow-brown patches on the otherwise light coloured surface of the basidiomata. However, these macro- and microscopic traits do not help to discriminate O. decimae from O. celatifilia (see below). Nonetheless, the ITS1-5.8S-ITS2 sequence of the holotype specimen of O. decimae (JN257991) was 88–93 % identical to the other species in subg. Parcaea in the BLAST search (Maximum identities; Query coverage = 89–95 %).
Yoshimi & Doi (1989) reported one Octaviania species from Amami-oshima Island, Japan, as O. tuberculata. We have examined this Japanese specimen (TNS-F-183206; Fig. 8g, h) and compared directly with the holotype specimen of O. tuberculata (NY780584; collected by R. Hesse, Oct. 1888 in Germany). The Japanese specimen clearly differed from the holotype in having smaller basidiospores, a smooth rather than verrucose peridium, and it was more similar to O. decimae in overall morphology. However, the size of basidiospores of the specimen has much wider range (8.4–18.6 × 7.4–15.7 μm, mean 11.9 × 10.7 [n = 35], SD: 2.71 [length] and 2.32 [width]) than that of typical O. decimae specimens. Since we have not been able to successfully extract DNA from this specimen and it differs morphologically from our other collections, we must tentatively identify the Japanese ‘O. tuberculata’ as an unknown species of subg. Parcaea.
Octaviania mortae Orihara, sp. nov. — MycoBank MB563168; Fig. 9a–e; Map 1
Etymology. Latin, Morta, is the name of one of the three Roman goddesses of fates, who cut the threads of human life, expressing metaphorically that this is one of the members of the cryptic species group.
Basidiomata solitary or in small clusters, up to 2.3 cm diam, subglobose to irregular tuberculate, firm, rubbery, sessile or with a concolorous reduced stipe at the base, surface glabrous, white to stramineous, gradually becoming dark grey to black when touched, rubbed or bruised, occasionally with sparse mycelial tufts concolorous with the peridial surface, rhizomorphs sparse, white, narrow, easily snapping off from the base of basidiomata. Peridium 0.3–2 mm thick when fresh, context white to stramineous, discolouration not observed. Gleba pale brownish when immature, becoming dark brown to blackish brown at maturity, structure very similar to O. nonae. Sterile base pulvinate or not developed, greyish white, sometimes connected to a short stipe, gradually turning blackish when exposed to air. Odour strong at maturity, unpleasant, a mixture of burnt and dimethyl sulfide smells.
Basidiospores globose to subglobose, spinose, dextrinoid, 11.1–(11.3−)14.9(−15.6) × 10.2–(10.4−)14.1(−15.6) μm, mean 13 × 12.1 μm (SD: 0.93 [length], 0.98 [width]), Q = 1–1.2, walls 1.2–2.3 μm thick, spinose ornaments 1.9(−3.6)–3.7 × 1.9–(2–4.2)–4.5 μm, mean 2.8 × 3.2 μm, pyramidal, internal cavities of ornaments slit-like in vertical section, labyrinthiform to zonate to cochleae in horizontal section. Basidia clavate to clavulate, hyaline, 2–4-spored, 22–31 × 10.1–13.7 μm, mean 25.2 × 11.4 μm, sterigmata up to 10 μm long. Basidioles clavulate, hyaline, almost the same size as basidia. Hymenia poorly developed. Subhymenium absent. Glebal trama of parallel to subparallel, non-inflated, thin-walled (up to 1 μm thick), white, filamentous hyphae 1.6–8 μm broad. Sterile base of mostly swollen, colourless, more or less interwoven, thin-walled (up to 0.8 μm thick) hyphae 3–25 μm broad; suprapellis of sterile base up to 50 μm thick, brown, composed of repent to somewhat trichodermial, more or less entangled, branched, non-inflated, pigmented, thin-walled (up to 0.8 μm thick) filamentous hyphae 2–9 μm broad. Peridium 150–1300 μm thick in dried specimens: peridiopellis up to 70 μm thick, pigmented in dark brown, of straight, non-inflated, thick-walled (1–2.2 μm thick), filamentous hyphae 2.5–10 μm broad subparallel to surface, forming a cutis; peridial context white, of densely interwoven, inflated, thin- to thick-walled (0.5–1.8 μm thick) filamentous hyphae intermingled with scattered, isodiametric to subspherical cells at maturity, 3–37 μm diam.
Habitat, Distribution & Season — Hypogeous or partially emergent under Castanopsis sieboldii, C. cuspidata, Quercus glauca and Q. acutissima Carruth.; Honshu and Shikoku (Japan); summer to autumn (July to November).
Holotype. Japan, Tottori Pref., Tottori-shi, Hie Shrine, under C. sieboldii, 16 July 2010, T. Orihara, KPM-NC0017774 (Orihara 1189; isotype TNS-F-41406).
Other specimens examined. Japan, Tottori Pref., Tottori-shi, Hie Shrine, under C. sieboldii, 8 Sept. 2008, T. Orihara, KPM-NC0017770 (Orihara 852); the same locality, 4 Oct. 2009, T. Orihara, KPM-NC0017772 (Orihara 1062); Kyoto Pref., Sakyo-ku, near Nanzen-ji temple, under C. cuspidata, 27 Oct. 2002, A. Kajiyama, KPM-NC0017769 (Orihara 218); the same locality, 1 Oct. 2009, A. Kajiyama, KPM-NC0017771 (Orihara 1044); Kyoto-shi, Kamikyo-ku, Kyoto-gyoen, under Q. glauca, 8 Nov. 2009, H. Saiki, KPM-NC0017773 (Orihara 1118) & TNS-F-41405 (duplicate); the same locality, 25 Oct. 2010, T. Orihara, KPM-NC0017775 (Orihara 1326); Ehime Pref., Futami-cho, in Quercus acutissima forest, 27 Sept. 2008, F. Nagao, KPM-NC0017724.
Notes — Although macroscopically it is quite difficult to discriminate O. mortae from the other members of subg. Parcaea, the species has several distinct microscopic features: short basidia (up to 31 μm long) and large basidiospores with large spiny ornaments (Table 3). The strong, burnt smell of the fresh, mature basidiomata is another diagnostic feature that can be determined in the field. Maximum identities of the ITS1-5.8S-ITS2 sequence with the other species of subg. Parcaea (i.e., O. nonae, O. decimae, and O. celatifilia) in the BLAST search were 89–93 % (Query coverage = 88–94 %).
Octaviania celatifilia Orihara, sp. nov. — MycoBank MB563169; Fig. 9f–k; Map 1
Etymology. The Latin words celatus (concealed) and filia (daughter) are combined. This epithet conveys the fact that this is a rare species that also belongs to subgen. Parcaea.
Basidiomata solitary or in small clusters, up to 1.2 cm diam, subglobose to depressed globose, firm, rubbery, sessile or with a concolorous rudimentary stipe at the base, surface glabrous, white at first, often with yellowish tint or patches, gradually becoming dark grey to black when touched, rubbed or bruised, rhizomorphs sparse, white, narrow, easily snapping off from the base of basidiomata. Peridium 0.2–0.8 mm thick when fresh, white, context white to cream colour. Gleba pale brownish when immature, becoming dark brown to blackish brown at maturity, structure similar to O. nonae. Sterile base pulvinate, greyish white to translucent to yellowish, often connected to a short stipe, gradually turning blackish when exposed to air. Odour unpleasant, a combination of sweet and dimethyl sulfide smells, similar to that of O. decimae.
Basidiospores globose to subglobose, spinose, dextrinoid, 9.9–12.9(−13.2) × (9.3−)9.4(−12)–12.1 μm, mean 11.4 × 10.8 μm (SD: 0.74 [length], 0.68 [width]), Q = 0.97–1.16, walls 1–2.2 μm thick, spinose ornaments 1.9(−3.6)–3.7 × 1.9–(2–4.2)–4.5 μm, mean 2.8 × 3.2 μm, conical to pyramidal, internal cavities of ornaments slit-like in vertical section, labyrinthiform to zonate to cochleae in horizontal section. Basidia clavate, hyaline, 2–4-spored, 23.8–36 × 8.8–13.2 μm, mean 29.9 × 10.9 μm, sterigmata up to 15 μm long. Basidioles clavate, hyaline, almost the same size as basidia. Hymenia poorly developed. Subhymenium absent. Glebal trama of subparallel, non-inflated, thin-walled (up to 0.6 μm thick), white, filamentous hyphae 2–7 μm broad. Sterile base of densely interwoven, narrow, white to colourless, thin-walled (c. 0.5 μm thick) filamentous hyphae 2.5–8 μm broad; suprapellis of sterile base up to 100 μm thick, pigmented in brown to ochraceous, composed of loosely entangled, septate, non-inflated, thin-walled (up to 0.6 μm thick) filamentous hyphae 2–7 μm broad. Peridium 300–600 μm thick in freeze-dried specimens; peridiopellis somewhat developed, up to 150 μm thick, pale ochraceous to pale reddish brown in colour, of straight, non-inflated, thin-walled (up to 1 μm thick), filamentous hyphae 3–8.5 μm broad subparallel to surface, forming a cutis; peridial context white to colourless, of dense, strongly inflated, cells up to 17.5 μm broad, walls up to 1.8 μm thick, but in immature basidiomata the context contains less inflated, interwoven filamentous hyphae.
No distinct difference from O. decimae found both morphologically and ecologically, but c. 90 positions in nuclear ITS rDNA sequence shown in Fig. 5 different from the latter species at the same level as the other species in subg. Parcaea.
Habitat, Distribution & Season — Hypogeous or partially emergent under Quercus gilva Blume; Honsyu (Nara Pref., Japan); early summer to winter (June to December).
Holotype. Japan, Nara Pref., Nara-shi, Nara Park, under Q. gilva, 3 Nov. 2010, H. Inui & T. Orihara, KPM-NC0017776 (Orihara 1337), holotype (isotype TNS-F-41407).
Other specimens examined. Japan, Nara Pref., Nara-shi, Nara Park, under Q. gilva, 25 June 2011, M. Ohmae, KPM-NC0018026 (Ohmae 140); Nara-shi, near Mt Kasuga, under Q. givla, 24 Dec. 2011, T. Orihara, KPM-NC018021.
Notes — This rare cryptic taxon has so far been found only in the two localities that are contiguous to each other. Moreover, its microscopic and macroscopic morphological similarities to O. decimae make identification extremely difficult: the only morphologically diagnostic character might be the slightly yellowish colour of its basidiomata. The yellowish tint, however, was not observed in every basidioma (Fig. 9f), and this colouration is also occasionally found in specimens of O. decimae. In addition, the habitat of O. celatifilia is similar to the other three species in subg. Parcaea. This species is found sympatrically with O. decimae: in one case they both occurred simultaneously within c. 400 m (Map 1). Therefore, the only reliable taxonomic key is unique DNA sequences which clearly distinguish it from the other three species in subg. Parcaea (Fig. 1, 2, 3, 4, 5). The BLAST maximum identities of the ITS1-5.8S-ITS2 sequence with the other three species in subg. Parcaea were 90–94 % (Query coverage = 91–95 %). In the future, the use of other DNA sequences will be helpful to more precisely understand the relationships between O. celatifilia and other species in subg. Parcaea.
Octaviania subgenus Fulvoglobus Orihara, subg. nov. — MycoBank MB563193
Typus subgeneris. Octaviania kobayasii Orihara.
Basidiomata hypogeous to emergent, rubbery or firm, surface whitish when immature, then usually tinged with yellowish, or becoming tawny to rusty at maturity. Peridium of two layers: peridiopellis thin, of mostly pigmented, filamentous hyphae; context of peridium of polygonal or subspherical cells comprising pseudoparenchyma, intermingled with partly inflated, filamentous hyphae. Oleiferous hyphae usually present, pigmented or colourless, context subgranulate.
Notes — Despite considerable phylogenetical divergence from the other clades in Octaviania, clade C (= subg. Fulvoglobus) has few unique morphological characters. We herein proposed the new subgenus for clade C based mainly on the characters of the peridial context and colour of the mature basidiomata as well as the dissimilarity of the nLSU rDNA and EF-1α sequences from that of the members of subg. Parcaea and Octaviania (Fig. 1, 2, 3). Species in subg. Parcaea are not tinged yellow overall but, similar to species in subg. Fulvoglobus, they do have a pseudoparenchymatous peridium at maturity. Subg. Octaviania is distinguished by the filamentous hyphae of its peridium that are not truly pseudoparenchymatous even at maturity.
Octaviania kobayasii Orihara, sp. nov. — MycoBank MB563194; Fig. 10
Fig. 10.
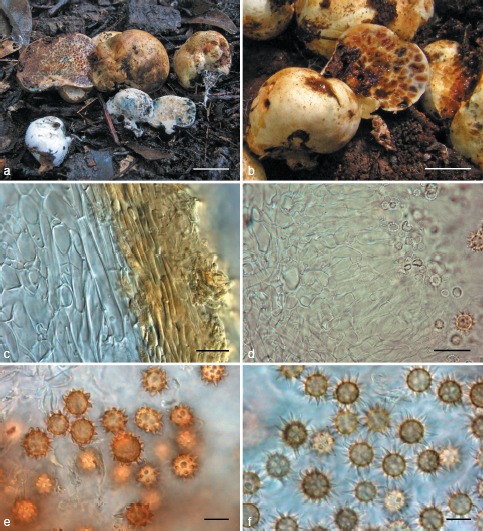
Octaviania kobayasii. a. Basidiomata (KPM-NC0017780); b. longitudinal section of basidioma (KPM-NC0017785); c. peridiopellis (right) and peridial context (left) (KPM-NC0017783); d. developed hymenial and subhymenial layers (KPM-NC0017785); e. basidiospores mounted in water (holotype); f. basidiospores mounted in lactic acid (holotype). — Scale bars: a = 1 cm; b = 5 mm; c, d = 20 μm; e, f = 10 μm.
Etymology. The epithet, kobayasii, was named in honour of Dr. Yosio Kobayasi, who greatly contributed to the taxonomy of hypogeous, sequestrate fungi in Japan, including Octaviania as well as other groups of fungi.
Basidiomata solitary to gregarious, up to 3.2 cm diam, subglobose, depressed-globose or tuberiform, rubbery, firm, with rudimentary stipe at the base, surface glabrous or sometimes partly cracked, exposing inner gleba, white in youth, then fulvous to tawny occasionally becoming gradually bluish green to blue, or wine red to reddish brown when touched, rubbed or bruised. Peridium up to 0.6 mm thick when fresh, context concolorous with the surface, turning a similar colour to the surface when exposed to air. Gleba pale brownish when immature, becoming ochraceous to fuscous at maturity, rubbery in youth, then becoming more or less brittle at maturity, composed of variously shaped chambers enclosed by glebal tramae forming a marbled pattern, spore mass becoming granulate at maturity or rarely somewhat glutinous; glebal trama of two strata: inner opaque, concolorous with the peridium, outer colourless, translucent. Sterile base rudimentary or slightly dendroid, concolorous with peridium, occasionally turning bluish green or red to reddish brown when exposed to air. Rhizomorphs white, easily snapping off from the base of basidiomata. Ectomycorrhiza bifurcate to plurifurcate, glabrous, light-brown to pale reddish brown. Odour nutty or like burnt sugar.
Basidiospores globose to broadly ellipsoid, spinose, weakly dextrinoid, pale ochraceous to tawny under the light microscope, (8.4−)8.6–13.6(−14.4) × (7.8−)8.1–12(−12.9) μm, mean 11.1 × 10.1 μm (SD: 1.23 [length], 0.96 [width]), Q = 0.98–1.45, walls 0.5–2 μm thick; ornaments spiny, relatively small, with a single cavity inside the wall, (1.1−)1.2–3.3 × (1.1−)1.2–3.6(−3.9) μm, mean 2.3 × 2.4 μm. Basidia clavate to cylindro-clavate, hyaline, evanescent, 2–4-spored, 19–38 × 8.2–11 μm, sterigmata up to 12 μm long. Cystidia absent. Hymenia developed, translucent, composed of basidia and interspersed basidioles. Glebal trama comprised of inner white and outer translucent tissues: white part of parallel or subparallel, partly inflated, thick-walled (0.5–1.5 μm thick), filamentous hyphae 2.5–10.5(−20) μm broad; outer, translucent part consisting of subhymenial cells inflated up to c. 40 μm diam and perpendicularly connected to each other from the inner white portion, walls of cells 0.5–1.2 μm thick. Sterile base of compact, densely interwoven, white filamentous hyphae 2.3–10 μm broad, walls 0.5–1.3 μm thick. Peridium 250–600 μm thick; peridiopellis up to 50 μm thick but absent in some patches, ochraceous, of repent, thin-walled (0.4–0.8 μm thick), filamentous hyphae 2.5–6.5 μm broad forming a cutis; peridial context white to pale ochraceous, of densely interwoven, more or less inflated, filamentous hyphae partly becoming isodiametric to subspherical (pseudoparenchymatous), 2.5–38 μm broad, hyphal walls thin in youth, becoming thick-walled (0.6–1.6 μm thick) at maturity.
Habitat, Distribution & Season — Hypogeous or subepigous under Castanea crenata Siebold & Zucc., Castanopsis sieboldii, Quercus dentata Thunb., Q. gilva, Q. serrata, and Pinus densiflora Siebold & Zucc.; Western Honshu (Japan); mid to late autumn (September to November).
Holotype. Japan, Okayama Pref., Maniwa-shi, Mt Minagasen, under Q. dentata and Castanea crenata, 15 Nov. 2008, T. Orihara, KPM-NC0017782 (Orihara 930; isotype TNS-F-41408).
Other specimens examined. Japan, Kyoto Pref., Kyoto-shi, Sakyo-ku, Shimogamo-hangi-cho, under C. sieboldii, 25 Sept. 2008, A. Kajiyama, KPM-NC0017779 (Orihara 887); the same locality, 29 Sept. 2008, T. Orihara, KPM-NC0017780 (Orihara 888); the same locality, 13 Dec. 2009, Y. Kitade, KPM-NC0017784 (Orihara 1125); Uji-shi, Taiyo-ga-oka Park, under P. densiflora, 28 Nov. 2009, F. Deai, KPM-NC0017783 (Orihara1122); Nara Pref., Nara-shi, Mt Kasuga, under Q. gilva, 3 Nov. 2010, H. Inui, K. Maruyama & T. Orihara, KPM-NC0017785 (Orihara 1342), TNS-F-41409 (duplicate) & KPM-NC0017786 (Orihara 1343; parasitized by Sepedonium chrysospermum); Hyogo Pref., Kobe-shi, Kita-ku, Yamada-cho, Shimo-tanigami, under Q. serrata, 9 Sept. 2006, T. Orihara, KPM-NC0017777 (Orihara 483); Akoh-shi, under Q. serrata, 10 Sept. 2006, T. Orihara, KPM-NC0017778 (Orihara 495); Okayama Pref., Maniwa-shi, Mt Minagasen, under Q. dentata and C. crenata, 12 Oct. 2008, F. Nagao, KPM-NC0017781 (Orihara 898); Hiroshima Pref., Kitahiroshima-cho, Higashiyawatahara, 13 Nov. 2010, M. Arita, KPM-NC0017787 (Orihara 1355); Ehime Pref., Kuma-kogen-cho, under Q. acutissima, 10 Nov. 2007, F. Nagao, KPM-NC0017723.
Notes — The most striking character of O. kobayasii is its thick, evanescent hymenial and subhymenial layers that look translucent macroscopically or under DIC microscopy. In addition, the dry and somewhat brittle spore mass at maturity is also diagnostic of O. kobayasii. Ecologically, this species tends to fruit in late autumn and is presumed to have weak host specificity since it occurs directly beneath deciduous and evergreen species of the Fagaceae (i.e., Quercus, Castanea, and Castanopsis spp.) as well as Pinus densiflora. Phenology is helpful for distinguishing O. kobayasii from O. hesperi (= Octaviania lineage C) because O. hesperi fruits in summer whereas O. kobayasii generally fruits in late autumn. Characters that differentiate O. kobayasii from other macroscopically similar species (i.e., O. japonimontana and O. etchuensis) are found in their descriptions below.
The EF-1α sequence of the specimen collected under Pinus densiflora (KPM-NC0017783) was slightly divergent from the other O. kobayasii sequences. This might reflect cryptic differences among isolates from different host plants (i.e., Pinus vs Fagaceae). However, the nLSU and ITS sequences between the Pinus-associated specimen and the other angiosperm-associated specimens were identical, and we could not find any obvious morphological differences. Although we refrain from proposing any infraspecific divisions within O. kobayasii, we recognize that there may be cryptic, host-specific diversity within this species.
Octaviania hesperi Orihara, sp. nov. — MycoBank MB563195; Fig. 6a, b, 11
Fig. 11.

Octaviania hesperi. a. Immature (centre) and mature (upper left) basidiomata (holotype). Upper right basidiomata are parasitized by Sepedonium chrysospermum; b. basidioma (section; KPM-NC0017795); c. peridium (KPM-NC0017791); d. basidia mounted in water (KPM-NC0017795); e. basidia with spores mounted in water (KPM-NC0017792); f. basidiospores mounted in lactic acid after presoaking in Melzer’s reagent showing weak dextrinoid reaction (KPM-NC0017792). — Scale bars: a = 1 cm; b = 5 mm; c = 30 μm; d–f = 10 μm.
Etymology. Latin, hesperi, after the Greek term hesperos (the evening star), referring to the large, ochraceous basidiospores with large, spiny ornamentation.
Basidiomata solitary or gregarious, up to 2 cm diam, subglobose, depressed-globose or tuberiform, rubbery, solid when immature, often with a rudimentary basal stipe, surface glabrous, sometimes becoming rimose-areolate at maturity forming an areolate pattern, white in youth, then stramineous to ochraceous, finally rusty at maturity, sometimes gradually becoming bluish green to indigo blue where touched, rubbed or bruised. Peridium up to 0.35 mm thick when fresh, concolorous with or paler than the surface, turning bluish green in some portions in cross section. Gleba whitish to pale brownish when immature, becoming fuscous to blackish brown at maturity, rubbery in youth, becoming glutinous at maturity, trama homogenous. Sterile base rudimentary to pulvinate, almost concolorous with the peridial context, occasionally turning bluish green to indigo blue when exposed to air. Rhizomorphs white, easily snapping from the base of basidiomata. Odour nutty.
Basidiospores globose to broadly ellipsoid, spinose, weakly to moderately dextrinoid, fulvous to tawny with light microscopy, 10–15.6(−18.2) × 9.4–(9.9−)14.8(−17.8) μm, mean 12.8 × 12.1 μm (SD: 1.40 [length], 1.31 [width]), Q = 0.96–1.15, occasionally with a pedicel 5.5–15 × 1.8–3 μm, pedicels sometimes with a ring-like appendage (Fig. 12f), spore walls 1.8–3 μm thick, ornaments spiny, 2–(2.1−)3.6(−4) × 1.3–(1.7−)5.4(−5.7) μm, mean 2.8 × 3.6 μm, large, polygonal at the base, with several large, distinct cavities that appear slit-like in vertical section and labyrinthiform to zonate to cochleae in horizontal section. Basidia clavate, hyaline, mostly 4-spored or more rarely 2–3-spored, 21.5–33.3 × 9.2–14.5 μm, sterigmata up to 15 μm long. Cystidia absent. Hymenia poorly developed, composed of basidia and interspersed basidioles up to 32 × 12.7 μm, deliquescent at maturity. Glebal trama of parallel to subparallel, white to more or less translucent, non-inflated or sometimes partly inflated, thin-walled (up to 0.8 μm thick), filamentous hyphae 2–8.5(−20) μm broad. Sterile base of compacted, densely interwoven, partly inflated, thin-walled, white filamentous hyphae 3–25 μm broad. Peridium 100–350 μm thick; peridiopellis up to 150 μm thick, absent in some portions, of straight, septate, more or less pigmented, partly inflated filamentous hyphae 2–10 μm broad forming cutis, hyphal walls 0.5–1.3 μm thick, outermost hyphae stained ochraceous to fuscous with light microscopy; peridial context 80–300 μm thick, almost colourless, of thick-walled (0.8–2 μm thick), spherical to subspherical, or isodiametric cells up to 92 μm diam intermingled with thin-walled (up to 1 μm thick), inflated filamentous hyphae 4–27 μm broad, but in youth the inflated cells are not yet developed and hyphal walls are thinner. Oleiferous hyphae present in peridium and trama, sinuate or monililform, pigmented, 2–9 μm broad.
Fig. 12.

Octaviania japonimontana. a, b. Basidiomata (a: KPM-NC0017798, holotype; b: KPM-NC0017801); c. basidiospores mounted in water (KPM-NC0017797); d. basidiospores mounted in 3 % KOH (KPM-NC0017800); e. 3-spored basidium (KPM-NC0017797); f. tips of oleiferous hyphae (holotype); g. hyphae of sterile base (KPM-NC0017797); h. peridiopellis (upper right) and context of peridium (holotype). — Scale bars: a, b = 1 cm; c–f = 10 μm; g, h = 50 μm.
Habitat, Distribution & Season — Hypogeous or subepigeous under Quercus glauca, Q. myrsinaefolia Blume, Castanopsis sieboldii or Lithocarpus edulis (Makino) Nakai, rare; Honshu (Japan); early summer to early autumn (June to October).
Holotype. Japan, Kanagawa Pref., Zushi-shi, Numama, under C. sieboldii, 21 July 2009, T. Orihara, KPM-NC0017793 (Orihara 984).
Other specimens examined. Japan, Ehime Pref., Hutami-cho, in Q. actissima forest, 27 Sept. 2008, F. Nagao, Nagao 08-09-12-08, KPM-NC0017727; Tokyo, Hachioji-shi, Matsugaya, Otsuka Park, under L. edulis, 26 Oct. 2008, T. Orihara, KPM-NC0017792 (Orihara 841); Kanagawa Pref., Zushi-shi, Numama, under C. sieboldii, Q. glauca, or Q. myrsinaefolia, 5 Sept. 2006, T. Orihara & M. Ohkubo, KPM-NC0017790 (Orihara 475); the same locality, 16 July 2007, T. Orihara, KPM-NC0017791 (Orihara 641) & TNS-F-41410 (duplicate); the same locality, under C. sieboldii, 21 July 2009, T. Orihara, KPM-NC0017794 (Orihara 985; parasitized by Sepedonium chrysospermum); the same locality, under C. sieboldii, 23 Oct. 2011, T. Orihara, KPM-NC0018019; Kyoto Pref., Kyoto-shi, Sakyo-ku, south-western foot of Mt Hiei, under Q. glauca, 27 Sept. 2005, T. Orihara, KPM-NC0017789 (Orihara 301) & TNS-F-41411 (duplicate); the same locality, 3 Oct. 2005, T. Orihara, KPM-NC0017788 (Orihara 300); Tottori Pref., Tottori-shi, Ouchidani, under C. sieboldii, 11 July 2010, T. Orihara, KPM-NC0017795 (Orihara1185).
Notes — Among members of subg. Fulvoglobus, O. hesperi (= Octaviania lineage C) is characterized by the relatively large basidiospores covered by large ornaments. In addition, the spore ornaments of this species contain multiple, distinct cavities. The macroscopically similar species, O. kobayasii is found in the same habitat as O. hesperi but the two species are microscopically distinct. In addition, O. kobayasii is readily distinguished from O. hesperi by its typically translucent hymenium and subhymenium as well as the tendency of O. kobayasii to from larger basidiomata.
Octaviania japonimontana Orihara, sp. nov. — MycoBank MB563196; Fig. 6c, d, 12
Etymology. Latin, japoni-, refers to its wide distribution within Japan, and Latin, montanus, refers to its mountainous habitat.
Basidiomata solitary to gregarious, subhypogeous or emergent, up to 4 cm diam, subglobose to reniform, rubbery, usually with a rudimentary basal stipe, surface glabrous to slightly floccose, often becoming partly areolate at maturity, white in youth, becoming stramineous to camel at maturity, gradually turning bluish green to cobalt blue, or red to wine, sometimes later turning blackish when touched, rubbed or bruised. Peridium varying in thickness, up to 0.7 mm thick when fresh, context paler than the surface, discolouring the same colour as the surface when exposed to air. Gleba whitish to pale brownish when immature, becoming fuscous to blackish brown at maturity, rubbery in youth, then becoming glutinous at maturity, glebal trama homogenous, concolorous with the peridial context. Sterile base rudimentary to pulvinate, concolorous with peridial context, discolouring the same colour as the surface when exposed to air. Rhizomorphs white, easily snapping off from the base of basidiomata. Odour nutty, strong at maturity.
Basidiospores globose to subglobose, spinose, dextrinoid, spadiceous to tawny with light microscopy, (9.1−)9.3–12(−12.1) × 8.7–(8.9−)11.3(−11.4) μm, mean 10.6 × 10 μm (SD: 0.67 [length], 0.64 [width]), Q = 0.93–1.21, with a basal appendage that has a ring-like edge, walls 1–2.4 μm thick; ornaments spiny, pyramidal, with one cavity inside the ornament wall, (1.5−)1.7–3.3(−3.5) × (1.2−)1.4–4.2(−4.3) μm, mean 2.5 × 2.8 μm. Basidia clavate, hyaline, 2–4-spored, 19–26.5 × 7–13 μm, mean 23.8 × 9.6 μm, sterigmata 2.5–9.5(−13) μm long and 1.5–2.3 μm broad. Oleiferous hyphae often present in the trama and the peridial context, filamentous, white to pigmented, more or less granulate, slightly swollen at hyphal tips. Hymenia poorly developed, composed of basidia and interspersed basidioles. Glebal trama of parallel to subparallel, non-inflated or sometimes partly inflated, white, thin-walled (up to 0.8 μm thick), filamentous hyphae 2–16 μm broad to surface. Sterile base of compacted, densely interwoven, partly inflated, thin-walled (0.4–0.8 μm thick), white filamentous hyphae 3.5–15 μm broad. Peridium 180–700 μm thick; peridiopellis 30–200 μm thick, usually well-developed, fulvous to pale brown near surface but whitish in colour toward the interior, of thin-walled (0.4–0.8 μm thick), filamentous hyphae 3–10 μm broad and parallel to subparallel to surface, forming a cutis; peridial context colourless, of densely interwoven, more or less inflated, filamentous hyphae 3–15 μm broad, some hyphal cells becoming subspherical or isodiametric, up to 70 μm diam at maturity; hyphal walls 0.7–1.6(−4) μm thick at maturity.
Habitat, Distribution & Season — Hypogeous or emergent under Fagus crenata Blume or Quercus crispula Blume; Honshu (Japan); summer to autumn (July to November).
Holotype. Japan, Tottori Pref.: Kofu-cho, Kagamiganaru, under F. crenata, 14 Oct. 2007, Y. Ando & T. Orihara, KPM-NC0017798 (Orihara 724; isotype TNS-F-41412).
Other specimens examined. Japan, Aomori Pref., Aomori-shi, Tashirodiara, under F. crenata and Q. crispula, 17 Aug. 2005, S. Kudo, KPM-NC0017799 (Orihara 771); Aomori-shi, Mt Hakkoda, under Q. crispula, 28 Aug. 2008, Y. Ando, KPM-NC0017802 (Orihara 859), KPM-NC0017803 (Orihara 860; parasitized by Sepedonium chrysospermum) & KPM-NC0017805 (Orihara 864; parasitized by Sepedonium sp.); Akita Pref., Kazuno-shi, Hakkatohge, near Lake Towada, 15 Sept. 2007, Y. Ando, KPM-NC0017797 (Orihara 700); the same locality, 28 Aug. 2008, Y. Ando, KPM-NC0017804 (Orihara 861) & TNS-F-41413 (duplicate); Aichi Pref., Kita-shitara-gun, Tengudana, under F. crenata, 6 Aug. 1983, S. Honda, KPM-NC0017796 (Orihara 219); Tottori Pref., Kofu-cho, Kagamiganaru, under F. crenata, 8 Sept. 2008, M. Nabe, KPM-NC0017808 (Orihara 955); the same locality, 12 Oct. 2008, S. Sato, KPM-NC0017807 (Orihara 896); Daisen-cho, Mt Daisen, Nino-sawa, in F. crenata forest, 12 Oct. 2008, S. Hirose, KPM-NC0017806 (Orihara 895); Yazu-cho, Hattoh, under F. crenata, 21 July 2008, S. Hirose, KPM-NC0017801 (Orihara 824); the same locality, 24 July 2008, T. Orihara, KPM-NC0017800 (Orihara 823); the same locality, 19 Aug. 2009, T. Orihara, KPM-NC0017809 (Orihara 1035) & KPM-NC0017810 (Orihara 1036); the same locality, 28 Aug. 2010, T. Orihara, KPM-NC0017811 (Orihara 1246); Okayama Pref., Kagamino-cho, Kami-onbara, under F. crenata, 25 Sept. 2010, T. Orihara, KPM-NC0017812 (Orihara1262) & TNS-F-41414 (duplicate).
Notes — So far, O. japonimontana has been found only under F. crenata or Q. crispula, the probable host trees for this species, in mountainous areas of Honshu. The morphologically similar species, O. kobayasii, O. hesperi, and O. etchuensis have not been found with these trees. Despite the morphological similarities between O. japonimontana and these other three species, our phylogenetic analyses clearly distinguish O. japonimontana as a unique taxon (Fig. 1, 2, 3). This is a good example of how host preferences might be used as a potential diagnostic feature among morphologically similar taxa.
The basidiospore morphology of O. japonimontana is also similar to European O. asterosperma, but the surface of O. japonimontana fruitbodies become tan to light brown at maturity whereas those of O. asterosperma do not. Also, peridial hyphae of O. asterosperma do not become pseudoparenchymatous as that of subg. Fulvoglobus even at maturity.
Octaviania sp. “E” — Fig. 13a–c
Fig. 13.
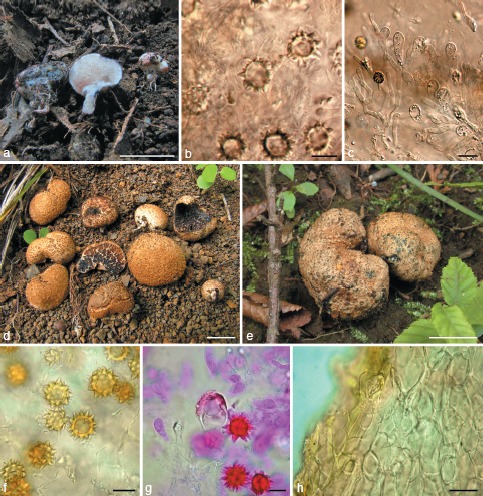
a–c: Octaviania sp. “E” (KPM-NC0017813): a. Immature basidiomata; b. immature basidiospores mounted in lactic acid after presoaking in 3 % KOH; c. poorly developed hymenium with basidioles. — d–h: O. durianelloides. d. Basidiomata (KPM-NC0017829, holotype); e. basidioma with green stains (KPM-NC0017815); f. basidiospores mounted in water (KPM-NC0017815); g. aberrant basidiospore connected directly to a basidium mounted in lactoglycerol after presoaking in 1 % phloxine B and 3 % KOH (centre; holotype); h. peridium (KPM-NC0017815). — Scale bars: a, d = 1 cm; b, f, g = 10 μm; c, h = 20 μm; e = 5 mm.
Only immature basidiomata were collected. Basidiomata solitary or in small clusters, hypogeous to subhypogeous, up to 1 cm diam, depressed-globose to tuberiform, rubbery, with a short, often rudimentary basal stipe; surface smooth, white when immature, turning red to dark reddish brown sometimes with an aeruginous tint when touched, rubbed or bruised, finally becoming blackish red to black. Peridium thin, up to c. 0.4 mm thick, white, discolouration not prominent. Gleba white in youth, gradually becoming dark brown, somewhat watery, discolouring the same colour as the surface when exposed to air, composed of variously shaped cells enclosed by glebal tramae forming a marbled pattern; glebal trama homogenous, whitish. Sterile base pulvinate, white. Rhizomorphs white.
Basidiospores globose to subglobose, spinose, dextrinoid, pale ochraceous to tawny under the light microscope, 9.1–(9.4−) 12.6(−13.1) × 8.5–(8.8)–11.5 μm, mean 10.8 × 10 μm (SD: 0.83 [length], 0.73 [width], n = 30, t = 2.045), Q = 1–1.28, walls 1.5–3.2 μm thick; ornamentation spiny, pyramidal, mostly with one cavity inside the wall, but exceptionally with several cavities, 1.3–(1.5−)3.8 × 1.3–(1.7−)4.1(−4.2) μm, mean 2.5 × 2.7 μm. Basidia clavate, hyaline, 2- or 4-spored, 21–36 × 8–11 μm, sterigmata 5.4–11 μm long. Oleiferous hyphae present in the tramal tissue and the peridial context, filamentous, pigmented, 2.5–4.5 μm diam. Hymenia poorly developed, composed of basidia and interspersed basidioles. Glebal trama of parallel or subparallel, colourless under DIC microscopy, non-inflated or sometimes partly inflated, thin-walled (up to 0.8 μm thick), filamentous hyphae 2–8 μm broad. Sterile base of compacted, densely interwoven, partly inflated, thin-walled (0.4–0.8 μm thick), white to translucent, filamentous hyphae 3.5–15 μm broad. Peridium 100–400 μm thick; peridiopellis 25–150 μm thick, more or less developed, stained with blackish red to black, of subspherical to isodiametric, thin-walled (up to 1 μm thick), cells up to 25 μm diam intermingled with more or less interwoven, partly inflated, pigmented, filamentous hyphae 4–13 μm broad; peridial context white to translucent under DIC microscopy, of densely interwoven, rarely inflated, filamentous hyphae 2–13 μm broad, mixed with subspherical or isodiametric cells up to 20 μm diam; hyphal walls up to 1 μm thick in youth.
Habitat, Distribution & Season — Hypogeous under Castanopsis sieboldii subsp. lutchuensis; Amami-oshima Island (the Ryukyu Archipelago, Japan); late autumn (November).
Specimen examined. Japan, Kagoshima Pref., Amami-oshima Isl., Yamato-son, north-eastern foot of Mt Yuwan, under C. sieboldii subsp. lutchuensis, 17 Nov. 2007, T. Orihara, KPM-NC0017813 (Orihara 761).
Notes — Unfortunately, the only Octaviania sp. “E” collection was immature and therefore inadequate for morphological evaluation and taxonomic delimitation. Thus, we leave it undescribed until mature specimens become available. The Octaviania sp. “E” specimen was found almost sympatrically with the holotype of O. nonae Orihara 945 in evergreen, subtropical forest on Amami-oshima Island in the northern Ryukyu Archipelago. Although these species are found in the same habitat, they are morphologically distinct and fall into different subgenera.
Octaviania durianelloides Orihara, sp. nov. — MycoBank MB563197; Fig. 6f, 13d–h
Etymology. The epithet, durianelloides, comes from the sequestrate fungus, Durianella echinulata (Corner & Hawker) Desjardin, A.W. Wilson & Binder, which is a macro-morphologically similar species. The strong odour of the basidiomata is also reminiscent of durian fruits.
Basidiomata solitary or in small clusters, subhypogeous or emergent, up to 2.5 cm diam, depressed-globose to reniform, rubbery, with a rudimentary basal stipe; surface covered with minute, somewhat fibrillar, felty warts or squamules, becoming wrinkled when dried, whitish to pale brown when immature, brown at maturity, gradually turning wine red when immature and dark green at maturity where rubbed or bruised, then later turning black to blackish green; tips of the peridial warts usually darker than the surrounding peridial surface. Peridium thin, only up to c. 0.4 mm thick when fresh, context white to beige, or somewhat translucent, discolouring dark blue when exposed to air at maturity. Gleba fuscous to black at maturity, rubbery in youth, then becoming somewhat glutinous at maturity, occasionally exuding water, composed of variously shaped cells; glebal trama homogenous, white to semi-transparent. Sterile base rudimentary to pulvinate, white, opaque. Rhizomorphs white. Odour slightly nutty or durian-like to unpleasant, with hints of dimethyl sulfide.
Basidiospores globose, subglobose, or broadly ellipsoid, spinose, dextrinoid, yellowish brown to fuscous under the light microscope, 8.1–(8.8−)15.1(−18.5) × 7.5–(8−)13.4(−15.1) μm, mean 11.6 × 10.4 μm (SD: 1.54 [length], 1.46 [width]), Q = 1–1.31, occasionally becoming aberrant and unusually swollen up to 26 μm diam and connected directly to basidia (Fig. 14g), walls 1–2(−2.5) μm thick; basal appendage not distinct; ornamentation spiny, acute, pyramidal, with one to several cavities inside each spore ornament wall, 1.3–3.9(−4.4) × 1.4–(1.5−)3.8(−4.1) μm, mean 2.6 × 2.6 μm, surface almost smooth under the light microscope. Basidia clavate to cylindro-clavate, hyaline, 2–4-spored, 17.5–35 × 8–11.8 μm, mean 25.7 × 9.7 μm (n = 12), often with sterigmata 5–10 μm long. Oleiferous hyphae not observed. Hymenia poorly developed. Glebal trama semi-transparent under DIC microscopy, of more or less loosely interwoven or subparallel, thin-walled (up to 1 μm thick), partly branched, filamentous hyphae 1.8–8.5(−10) μm broad, intergrading with spherical cells up to c. 30 μm diam. Sterile base white under DIC microscopy, collapsed in dried specimens and not rehydrated when soaked in water or 3 % KOH. Peridium 200–400 μm thick; peridiopellis 40–100(−150) μm thick, yellow-brown, of partly branched, pigmented filamentous hyphae 2.8–13 μm broad but partly inflated to 23 μm broad, parallel to subparallel to surface, forming a cutis, walls 0.5–1.8 μm thick; peridial context 100–325 μm thick, of colourless, mostly of interwoven, inflated, thick-walled (0.4–2.2 μm thick), filamentous hyphae 3–10.5(−18) μm broad, becoming mostly pseudoparenchymatous up to 35 μm diam at maturity.
Fig. 14.
a–f: Octaviania yaeyamaensis (KPM-NC0017819, holotype). a. Basidiomata; b. peridium; c. tramal hyphae; d. basidiospores mounted in water; e. basidiospores mounted by lactoglycerol after presoaking in 3 % KOH; f. basidia. — g–l: O. etchuensis (KPM-NC0017822, holotype). g. Basidiomata; h. peridium; i. context of peridium; j. basidiospores mounted in water; k. basidiospores mounted by lactoglycerol after presoaking in 3 % KOH; l. trama. — Scale bars: a, g = 1 cm; b, h, l = 50 μm; c, i = 20 μm; d–f, j, k = 10 μm.
Habitat, Distribution & Season — Under Castanea crenata, Quercus serrata, or Q. acutissima; central to northern Honshu (Japan); early to mid summer (June to August).
Holotype. Japan, Kanagawa Pref.: Minami-ashigara-shi, Uchiyama, under C. crenata, 4 July 2011, T. Orihara, KPM-NC0017829 (Orihara 1386; isotype TNS-F-41415).
Other specimens examined. Japan, Miyagi Pref., Shichigasyuku-cho, under Q. serrata, 23 July 2004, Yoko Ando, KPM-NC0017814 (Orihara 148); Sendai-shi, Izumi-ku, eastern foot of Mt Kurohana, 28 July 2006, H. Sasaki, KPM-NC0017816 (Orihara 1170); Saitama Pref., Kawagoe-shi, under Q. serrata and Q. acutissima, 27 June 2006, Ikuo Asai, KPM-NC0017815 (Orihara 410); Shiga Pref., Hino-cho, Kamagake, 23 Aug. 2007, Y. Kotera, KPM-NC0017817 (Orihara 1377).
Notes — Octaviania durianelloides is unique in Octaviania because of its warty, brown peridium and dark green discolouration where injured, but otherwise it is morphologically similar to the other species in the genus. The macromorphology of O. durianelloides is similar to Durianella echinulata (syn. Hydnangium echinulatum; Boletales, Boletineae), which was reported from Malaysia and forms greyish orange to golden yellow, durian-shaped basidiomata (Desjardin et al. 2008). However, D. echinulata differs from O. durianelloides in having larger and more distinct peridial warts (up to 1 mm tall), thicker peridium (1–2 mm thick), and basidiospores covered with collapsible spines. Moreover, rDNA LSU sequences of D. echinulata EU293063 and O. durianelloides were only c. 86 % similar, indicating that these taxa are not close relatives. Thus, the superficial morphological similarities between these two sequestrate fungi is due to convergent evolution within Boletales. The acute ornamentation of basidiospores and relatively wide range of the basidiospore size, some becoming unusually swollen, are additional diagnostic features of O. durianelloides.
Octaviania yaeyamaensis Orihara, sp. nov. — MycoBank MB563198; Fig. 14a–f; Map 1
Etymology. The epithet refers to the known distribution of the species. The type locality, Ishigaki Island, is one of the Yaeyama Islands, which makes up the southernmost part of the Ryukyu Archipelago, Japan.
Basidiomata solitary to gregarious, hypogeous to subhypogeous, up to 3 cm diam, subglobose, depressed globose or reniform, firm, rubbery, sessile or with rudimentary stipe at the base, surface smooth to slightly tomentose, white when young, then becoming yellowish white to pale stramineous with a reddish brown or bluish green tint at maturity, turning red to reddish brown or bluish green where bruised, with whitish brown rhizomorphs at the base. Peridium well developed, 0.2–1 mm thick, rubbery, often gradually turning red to reddish brown when cut. Gleba cream-coloured to pale brown when young, then blackish brown to chocolate brown at maturity, glebal chambers filled with numerous basidiospores immersed in a glutinous matrix, trama white at first, but quickly turning indigo blue to blackish blue at maturity when exposed to air. Sterile base pulvinate or somewhat dendroid, upper portion turning the same colour as glebal trama at maturity when exposed to air, the lower portion turning the same colour as peridium. Odour pleasant, slightly sweet, refreshing, similar to pandan leaves (Pandanus amaryllifolius Roxb.).
Basidiospores globose to subglobose, weakly dextrinoid, spinose, 9.3–(9.5−)14.8(−16.6) × 8.4–(9.1−)13.9(−14.4) μm, mean 12 × 11.2 μm (SD: 1.34 [length], 1.33 [width]), Q = 0.96–1.23, walls 1–2.3 μm thick, with pedicel up to 10 μm long at the base, surface covered with dense, relatively small, conical to polygonal spines with a single cavity inside the walls of each ornament, 12–16 spines per circumference, 0.9–(1−)2.6(−3.3) × (1.3−)1.4–3(−3.2) μm, mean 1.7 × 2.2 μm in water. Basidia clavate, 2- or 4-spored, 23–33 × 8–12.5 μm, mean 27.6 × 10.5 μm, evanescent, hyaline, sterigmata 3.5–12 μm long. Cystidia absent. Subhymenium absent. Hymenia poorly developed, composed of basidia and interspersed basidioles. Trama of white, thin-walled (up to 0.8 μm thick), filamentous hyphae 2.5–9 μm broad, more or less parallel to hymenium. Sterile base of densely interwoven, white to ochraceous, thin-walled (up to 1 μm thick), partly inflated, filamentous hyphae 3–15 μm broad. Peridium well developed, 400–900 μm thick when fresh; peridiopellis 70–160 μm thick, of repent, thin-walled (up to 1 μm thick), non-inflated filamentous hyphae 2.5–13.2 μm broad, subparallel to surface, hyphae in outermost portion pigmented ochraceous-brown; peridial context white under the DIC microscope, composed of subspherical, thick-walled (0.9–2.7 μm thick), inflated cells up to 78 μm diam intermingled with inflated, thin-walled (up to 1.2 μm thick), filamentous hyphae 4–25 μm broad often filled with oleiferous, intracellular pigments.
Habitat, Distribution & Season — Under Castanopsis sieboldii subsp. lutchuensis; Ishigaki Isl.; early winter (December).
Holotype. Japan, Okinawa Pref., Ishigaki Isl., Ishigaki-shi, on southern foot of Mt Omoto, 18 Dec. 2009, T. Orihara, KPM-NC0017819 (Orihara 1137; isotype TNS-F-41416).
Another specimen examined. Japan, Okinawa Pref., Ishigaki Isl., Ishigaki-shi, on southern foot of Mt Omoto, 12 Dec. 2009, T. Orihara, KPM-NC0017818 (Orihara 1127).
Notes — The subtropical O. yaeyamaensis shares similar microscopic characters (i.e., medium-sized basidiospores surrounded by relatively small spines that each have only a single cavity) and habitat with Octaviania sp. “E”, which was collected from Amami-oshima Island, about 700 km northeast from Ishigaki Island. Molecular data, however, confirm that they were distinct species in the same subgenus (Fig. 1, 2, 3). Octaviania yaeyamaensis is also morphologically similar to O. malaiensis (Corner & Hawker) Pegler & T.W.K. Young, which is one of the few known tropical species. Both species have whitish peridia, distinctly cyanescent glebae, and small spiny basidiospore ornaments with single cavities. However, O. malaiensis differs in its smaller basidiospores (i.e., mostly less than 10 μm diam) and basidiospore ornaments (i.e., less than 1.6 μm high in water). The combination of a rubbery, developed peridium that tinged more or less yellowish at maturity in combination with the pandan-like odour of the mature basidiomata is characteristic to O. yaeyamaensis.
Octaviania etchuensis Orihara, sp. nov. — MycoBank MB563199; Fig. 14g–l; Map 1
Etymology. The epithet refers to the old name for Toyama Prefecture, Japan, where the holotype specimen was collected.
Basidiomata solitary or in small clusters, subhypogeous, up to 1.5 cm diam, subglobose to depressed globose, firm, rubbery, often with a short stipe up to 4 mm high at the base, surface smooth or partially with concolorous mycelial tufts, whitish in youth, dirty yellow to fulvous with reddish brown tint at maturity, turning bluish green to blackish where touched or bruised, with whitish dirty yellow to light-yellow rhizomorphs at the base. Peridium 0.3–1 mm thick when fresh, rubbery, context white. Gleba pale brown when immature, then blackish brown at maturity, glebal chambers mostly filled with masses of basidiospores and immersed in a glutinous matrix, trama white, turning bluish green and sometimes finally turning blackish when cut. Sterile base pulvinate or somewhat dendroid, beige, occasionally turning blackish when exposed to air. Odour faint, pleasant, similar to that of O. yaeyamaensis.
Basidiospores globose to broadly ellipsoid, weakly dextrinoid, spinose, 9.3–(9.4−)12.3(−12.8) × 8.1–10.8(−11.6) μm, mean 10.8 × 9.4 μm (SD: 0.75 [length], 0.67 [width]), Q = 1.04–1.15–1.36, walls 1–2 μm thick, with pedicel up to 6 μm long at the base, surface covered with dense, relatively small, low, polygonal spines with single cavity inside the walls, 14–21 spines per circumference, 0.8–2.4 × (1−)1.1–2.9(−3) μm in water, mean 1.6 × 2 μm. Basidia clavate, colourless, 2- or 4-spored, evanescent, mostly collapsed. Cystidia and subhymenium not seen. Hymenia poorly developed, composed of basidia and interspersed basidioles. Trama colourless with light microscopy, composed of densely interwoven to subparallel, non-inflated, thin-walled (up to 0.8 μm thick), filamentous hyphae 2–7.5 μm broad, hyphae in fork of tramal plates loosely interwoven, filamentous. Sterile base of densely interwoven, colourless to slightly brownish, non-inflated filamentous hyphae 3.5–10(−14) μm broad, walls c. 1 μm thick. Peridium 200–750 μm thick in dried specimen; peridiopellis up to 150 μm thick, ochraceous to golden-yellow at surface, inner paler to colourless, of narrow, non-inflated filamentous hyphae 1.8–6.5 μm broad, partially inflated to 25 μm diam, parallel to subparallel to surface, hyphae in outermost portion strongly pigmented, walls up to 1.3 μm thick; peridial context up to 600 μm thick, colourless to slightly brownish, of densely interwoven to subparallel, filamentous hyphae when immature, then becoming darker and pseudoparenchymatous with cells up to 35 μm diam at maturity intermingled with narrower, thick-walled, filamentous hyphae, mature cell walls up to 3 μm thick, oleiferous hyphae not observed.
Habitat, Distribution & Season — Subhypogeous in Quercus serrata and Pinus densiflora forests, rare; Honsyu (Japan); autumn (September to November).
Holotype. Japan, Toyama Pref., Nakakniikawa-gun, Tateyama-machi, Tochizu, in Q. serrata and Pinus densiflora forest, 16 Sept. 2001, M. Hashiya, KPM-NC0017822 (Orihara 368; split: Hashiya 2785; isotype TNS-F-41417).
Other specimens examined. Japan, Toyama Pref., Nakakniikawa-gun, Tateyama-machi, Tochizu, in Q. serrata and Pinus densiflora forest, 18 Sept. 2000, T. Ohara, KPM-NC0017821 (Orihara 367; split: Hashiya 2449); Kyoto Pref., Sonobe-cho, Ruri-kei, 11 Nov. 2001, A. Kajiyama, KPM-NC0017820 (Orihara 214); Hyogo Pref., Kobe-shi, Yamada-cho, Shimotanigami, under Q. serrata, 24 Sept. 2011, S. Koutoku, KPM-NC0017849.
Notes — Octaviania etchuensis is a rare species that is closely related to O. yaeyamaensis. Together these species constitute a unique monophyletic group within subg. Fulvoglobus (Fig. 1, 2, 3). Morphologically, both of these species have firm, moderately developed peridia, reduced appendages on the pedicels, and small spiny ornaments on their basidiospores. However, O. etchuensis has only been collected in temperate forests containing species of the deciduous Fagaceae and Pinus species whereas O. yaeyamaensis is known from subtropical forests with Castanopsis sieboldii subsp. lutchuensis. Morphologically, O. etchuensis has slightly smaller basidiospores than O. yaeyamaensis. In addition, O. etchuensis also has a more yellowish peridium that turns bluish green to blackish when touched or bruised whereas the peridium of O. yaeyamaensis has other staining reactions but does not turn black. The basidioma colour and the basidiospore dimensions of O. etchuensis are almost the same as those of O. kobayasii, but the subhymenial layer of the latter species is translucent and more developed, and the basidiospore ornaments of this species are larger than those of O. etchuensis.
Octaviania subgenus Octaviania
Typus subgeneris. Octaviania asterosperma Vittad.
Basidiomata hypogeous to subepigeous, soft, surface smooth to floccose to rimose, surface white when immature, then off-white to fuligineous grey at maturity, turning red to reddish brown or bluish green to cyaneous and finally blackish when injured. Peridium mostly thin, not becoming rubbery, context of thin-walled, non-inflated or partly inflated filamentous hyphae usually not exceeding 20 μm broad.
Notes — Subgenus Octaviania is an autonym that results from the establishment of subg. Parcaea and Fulvoglobus. This subgenus is equivalent to Octaviania clade B, which includes O. asterosperma, O. cyanescens, O. zelleri (= O. nigrescens (Zeller) Singer & A.H. Sm.), O. tasmanica, and O. asahimontana. The nLSU and multigene phylogeny indicates that the subgenus also includes other European Octaviania species that are morphologically similar to O. asterosperma (Fig. 1, 3). However, we so far refrain from concluding their subgeneric position since most of them have not been reported for more than 50 years and their comprehensive molecular phylogeny is still infeasible until their reliable specimens are newly collected.
Octaviania zelleri Orihara & M.E. Sm., nom. nov. — MycoBank MB564118
≡ Hydnangium nigrescens Zeller, Mycologia 40, 6: 641, 1948.
= Octaviania nigrescens (Zeller) Singer & A.H. Sm., Mem. Torrey Bot. Club 21, 3: 11. 1960; non J.W. Cribb, Papers of the Department of Botany, University of Queensland 3: 249. 1958 (as ‘Octavianina nigrescens’).
Etymology. The epithet is named in honour of the authority of the basionym, Prof. Sanford Myron Zeller (1885–1948), who significantly contributed to taxonomy of sequestrate and gasteroid fungi.
Notes — The species was first described by Zeller (1948) as Hydnangium nigrescens based on a specimen collected from New York, USA. The species was later transferred to Octaviania by Singer & Smith (1960). However, Cribb (1958) had already proposed the name O. nigrescens based on a different type collected from Queensland, Australia. Thus, Octaviania nigrescens (Zeller) Singer & A.H. Sm. is nomenclaturally illegitimate. Although the taxonomic treatment of this species was not a main objective of this publication, we consider this nomenclatural revision necessary in order to clarify the taxonomy of the genus Octaviania. Accordingly, we herein propose a substitute name, O. zelleri, for this taxon.
Octaviania asahimontana Orihara, sp. nov. — MycoBank MB563200; Fig. 15
Fig. 15.
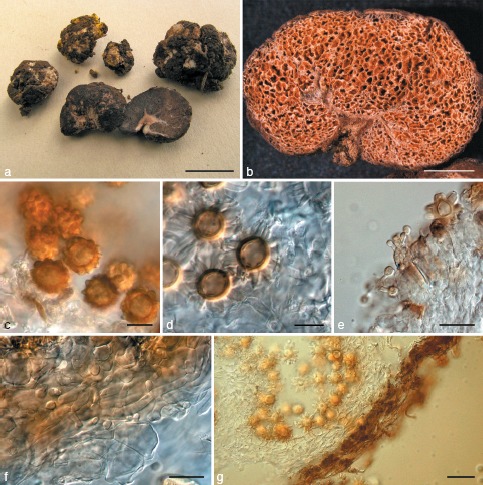
Octaviania asahimontana. a. Basidiomata (KPM-NC0017825). The yellow basidiomata are parasitized by Sepedonium chrysospermum; b. longitudinal section of air-dried basidioma (KPM-NC0017823, holotype); c. basidiospores mounted in water (holotype); d. basidiospores mounted in lactoglycerol after presoaking in 3 % KOH (KPM-NC0017825); e. basidia (KPM-NC0017824); f. tramal hyphae and inflated cells (KPM-NC0017824); g. peridium mounted in lactoglycerol after rehydration with 3 % KOH.
Etymology. The epithet referring to the type locality for the species, Mt Daisetsu, which is located in the middle of Hokkaido. Mt Asahi-dake is a part of Mt Daisetsu.
Basidiomata solitary to gregarious, hypogeous to subhypogeous, up to 2.5 cm diam, subglobose to tuberculate, soft, more or less fragile, sessile, surface smooth to slightly tomentose, pure white to greyish white, turning red when touched or bruised, finally becoming dark reddish brown to black. Peridium not well developed, very thin but persistent. Gleba cream-coloured to pale brown when young, then blackish brown at maturity, glebal chambers minute (< 0.2 μm diam), with only a minimal glutinous matrix, trama whitish in thick portion but somewhat translucent in the narrower portion, the narrow portion of trama gradually turning blackish when exposed to air. Sterile base usually well developed, dendroid, white to pale whitish brown and columella-like. Rhizomorphs white. Odour unknown.
Basidiospores globose to broadly ellipsoid, weakly dextrinoid, warty to spiny, brown at maturity, 12.6–(13−)17.1(−17.7) × 11.3–15.6(−15.8) μm, mean 14.9 × 13.4 μm (SD: 1.11 [length], 1.07 [width]), Q = 0.95–1.27, walls 1.8–3 μm thick but exosporium gradually swollen up to 3.5 μm thick when mounted in acidic or alkaline solutions, surface densely covered with large, polygonal spines 1.5–3.7(−3.8) × 2.2–(2.4−)5.5(−6.4) μm, mean 2.6 × 3.9 μm with 1–3 cavities inside each spore ornament wall. Basidia clavulate to doliiform, 2–4-spored, hyaline, evanescent, 17–30 × 8.5–14.2 μm, mean 24 × 11.5 μm, sterigmata 4–10 μm long. Hymenium composed of basidia and clavulate, doliiform or subspherical basidioles. Trama of white, thin-walled (up to 0.8 μm thick), non-inflated filamentous hyphae 2.5–6(−7) μm broad with translucent, polygonal to subspherical cells up to 33 μm diam, with bundles of parallel, filamentous hyphae running in the centre of the tramal plates. Peridium thin, 30–200 μm thick, composed of only one layer, of thin-walled (up to 0.8 μm thick), white to ochraceous, filamentous hyphae 2–7 μm broad, hyphae almost congeneric with the tramal hyphae. Oleiferous hyphae often present within tramal tissue and peridial context, sinuate, reddish brown, partly inflated, 3.5–9 μm broad. Clamp connections absent in all tissues.
Habitat, Distribution & Season — Under Betula ermanii Cham. in the mountain areas in central Hokkaido (Japan); summer (August).
Holotype. Japan, Hokkaido, Kamikawa-cho, on the mid-slope of Mt Daisetsu, 16 Aug. 2006, S. Sato, KPM-NC0017823 (Orihara 501), holotype (isotype TNS-F-41418).
Other specimens examined. Japan, Hokkaido, Kamikawa-cho, on the mid-slope of Mt Daisetsu, 10 Aug. 2009, S. Sato, KPM-NC0017824 (Orihara 1023); the same locality, 12 Aug. 2009, S. Sato, KPM-NC0017825 (Orihara 1024) & KPM-NC0017826 (Orihara 1027; parasitized by Sepedonium chrysospermum).
Notes — Among Japanese Octaviania spp., O. asahimontana is unique in having a well-developed, dendroid sterile base (columella) and clavulate to doliiform basidia as well as its association with Betula trees in the boreal region. Two morphologically similar European species, O. lanigera Hesse and O. lutea Hesse are also collected under Betula trees in Germany. Octaviania lanigera, however, is morphologically distinct from O. asahimontana in having an undeveloped, pulvinate sterile base and a thicker peridium (nearly 1 mm) (Dodge & Zeller 1936, Singer & Smith 1960). We observed an authentic specimen of O. lanigera (NY780603) that was collected by R. Hesse in Germany 10 years after the holotype description, and confirmed that its basidiospores were also smaller than O. asahimontana (mean 11.8 × 10.6 μm excl. ornamentation, n = 15). Basidiospores of an authentic O. lutea specimen collected by R. Hesse (NY780607) were almost identical to that of O. lanigera and, thus were also smaller than basidiospores of O. asahimontana (mean 12.5 × 11.3 μm excl. ornamentation, n = 12). Octaviania cyanescens Trappe & Castellano, the probable sister species of O. asahimontana (Fig. 3, 4), has similar basidiospore morphology to O. asahimontana but differs in having strongly cyanescent basidiomata and a gleba filled with a blackish brown spore mass.
The almost non-glutinous glebal cavities and the basidiospore walls that become swollen when mounted in acidic or alkaline solutions, are reminiscent of the genus Heliogaster, another morphologically similar, but independent sequestrate genus (Orihara et al. 2010). Phylogenetic analyses clearly indicate that these similarities were the result of convergent evolution and also clarified that O. asahimontana is nested within the subclade B with Australian, European, and North American taxa and not with other Japanese taxa (Orihara et al. 2010 and this study). This suggests a biogeographical history that is independent from the other Japanese species.
DOUBTFUL SPECIES
Octaviania asterosperma sensu Minakata ex Kobayasi, Otani, & Hagiw., Fungal enumeration by Kumagusu Minakata 1: 92. 1987; non Vittad., Monogr. Tuberac.: 17. 1831.
In November 1932, one basidioma of an Octaviania species was collected in Iwatamura, Kii (known today as Wakayama Prefecture). Mr. Kumagusu Minakata examined the basidioma and named it Octaviania atrovirens Minakata nom. inval., leaving a colour sketch and description (Kobayasi et al. 1987). Kobayasi et al. (1987) later examined the sketch and description and identified the specimen as O. asterosperma. Undoubtedly, however, this description is insufficient to accurately identify the specimen (i.e., the description is based on only one basidioma and lacks microscopic observations), but it is not likely conspecific with the holotype of O. asterosperma. The yellowish tinge and bluish green stains mentioned in the description indicate that the specimen probably belongs to subg. Fulvoglobus and could represent either O. kobayasii or O. hesperi. However, since we have not been able to examine the specimen, its identity remains uncertain.
Key to the Japanese species of Octaviania (with notes on morphologically similar taxa from other world regions)
1. Basidiomata whitish, occasionally with an yellowish tinge, finally turning black when touched or bruised, peridium thick, usually exceeding 0.4 mm thick, occurring under Castanopsis or evergreen Quercus spp., basidiospore ornamentation containing several longitudinal cavities inside the walls ..................... Subg. Parcaea (see Table 3, Fig. 4, 5)
1. Basidiomata becoming yellowish to brown at maturity, or turning reddish or bluish green when touched or bruised, found beneath trees in the Fagaceae or Betulaceae ..................... 2
2. Basidiomata off-white to fuligineous grey at maturity. Peridium composed of thin-walled, filamentous hyphae, thick-walled pseudoparenchymatous hyphae absent ..................... 3 (Subg. Octaviania)
2. Basidiomata firm or rubbery, becoming more or less yellowish or brownish at maturity, found beneath trees in the Fagaceae. Peridial context of inflated, isodiametric to polygonal hyphae in most part at maturity, becoming pseudoparenchyma ..................... 6 (Subg. Fulvoglobus)
3. Average size of basidiospores exceeding 14 μm diam. — Japan or North America ..................... 4
3. Average size of basidiospores not exceeding 14 μm diam. — Europe ..................... 5
4. Basidiomata fragile, not becoming deep blue when rubbed or bruised, found under Betula sp. Sterile base more or less developed becoming a dentroid columella ..................... O. asahimontana
4. Basidiomata firm, distinctly cyanescent, found under conifers (e.g., Tsuga mertensiana). Sterile base reduced, indistinct ..................... O. cyanescens (North American species phylogenetically close to O. asahimontana)
5. Ornaments of basidiospores mostly contain one, simple cavity. Basidiomata found beneath trees in the Fagaceae ..................... O. asterosperma (the generic type species)
5. Ornaments of basidiospores contain multiple (2–6) cavities that look labyrinthiform to zonate to cochleae in horizontal section. Basidiomata found under trees in the Betulaceae O. lanigera / O. lutea species complex (the European group that needs taxonomic re-evaluation based on molecular data)
6. Peridium well developed, exceeding 0.8 mm thick in some portions. Basidiospore ornaments small, mostly less than 2.6 × 3 μm in water (on average less than 2 μm high), with a single cavity inside each spore ornament. Appendages on the basidiospore pedicels reduced and indistinct ..................... 7
6. Peridium thin, less than 0.7 μm thick. Basidiospore ornaments larger, with average height in water exceeding 2 μm. Appendages on the basidiospore pedicels somewhat developed and usually visible under light microscopy at × 1000 ..................... 9
7. Average size of basidiospores less than 10 μm diam ..................... O. malaiensis (species known from the Malay Peninsula)
7. Average size of basidiospores exceeding 10 μm diam ..................... 8
8. Basidiomata not staining yellow. Average size of basidiospores 12 × 11.2 μm (Q = 0.96–1.23). — The Yaeyama Islands (the south-western Ryukyu Archipelago) ..................... O. yaeyamaensis
8. Basidiomata pale yellow to ochraceous at maturity. Average size of basidiospores 10.8 × 9.4 μm (Q = 1.04–1.36). — Honsyu (the mainland of Japan) ..................... O. etchuensis
9. Surface of peridium warty or scaly, brown, turning dark green when bruised ..................... O. durianelloides
9. Surface of peridium smooth to slightly floccose, turning reddish or bluish green when bruised ..................... 10
10. Average size of basidiospores exceeding 12 μm diam, basidiospore ornaments large, containing multiple cavities in each spore ornament that are slit-like in vertical section and labyrinthiform to zonate to cochleae in horizontal section ..................... O. hesperi
10. Average size of basidiospores not exceeding 12 μm diam. Basidiospore ornaments less than 4.5 μm in width, usually containing 1–3 cavities in each spore ornament that are simple to curved in horizontal section ..................... 11
11. Glebal trama consisting of two distinct layers: inner layer white to cream colour and composed of filamentous hyphae; outer layer translucent, of developed hymenia and subhymenia ..................... O. kobayasii
11. Glebal trama of a single, whitish to cream coloured layer ..................... 12
12. Basidiomata soft, occurring under Castanopsis sieboldii subsp. lutchuensis. — Amami-oshima Island (the northern Ryukyu Archipelago) ..................... Octaviania sp. “E”
12. Basidiomata firm, rubbery, occurring under Fagus crenata or Quercus crispula. — Mainland of Japan ..................... O. japonimontana
DISCUSSION
In this study we have proposed 11 new species and 2 subgenera (plus 1 autonym) in the genus Octaviania. At least one additional species (i.e., Octaviania sp. “E”) was shown to be phylogenetically unique but will await formal description until mature specimens can be obtained and studied. The description of 11 species from Japan constitutes an approximately 60 % increase in the number of accepted Octaviania species to c. 30 species (Kirk et al. 2008 and see introduction of this paper). Since the Japanese Archipelago has partially and repeatedly been connected to the continent until the late of the Pleistocene (Fujii 1990), these findings suggest that the genus Octaviania is probably highly diverse in Asia. It is likely that many more species remain to be described, particularly from Asian tropical and subtropical habitats where a few unique Octaviania species have already been reported (Corner & Hawker 1953, Pegler & Young 1979).
All of the phylogenetic analyses in this study recovered the three highly divergent clades within the genus Octaviania (clades A–C; Fig. 1, 2, 3). Clade A is morphologically and ecologically distinct and we have proposed a new subgenus Parcaea for this group. The other two have fewer distinct diagnostic morphological characters, despite the significant phylogenetic divergence among the three subgenera. One important character that differentiates subg. Octaviania is the peridial context that consists of thin-walled filamentous hyphae and does not become truly pseudomarenchymatous at maturity. In contrast, subg. Fulvoglobus is characterized by basidiomata that become yellowish to tawny to rusty at maturity and a peridial context that consists of inflated hyphae intermingled with pseudoparenchymatous cells. However, immature specimens are very difficult to identify morphologically to the species level. Therefore, other methods that are applicable to identification of specimens at any stage, such as chemotaxonomical approaches as well as molecular techniques, would be helpful to facilitate subgenus-level delimitation in the genus Octaviania.
At the species level, we found few morphological characteristics that can be routinely used to discriminate among the Japanese Octaviania spp. Variations in the ultrastructure of cavities inside basidiospore ornaments is informative overall, but these characteristics are not always distinctive. It is possible, in most cases, to morphologically distinguish similar species by a combination of spore ornaments, basidiospore size, and habitat requirement or ectomycorrhizal host plant association. It should be noted here that the presence of cavities is not a character unique to the genus Octaviania (Orihara et al. 2010).
The phylogenetic analyses of the nLSU and combined dataset showed two different lineages of O. asterosperma (Fig. 1, 3). DNA sequences from the Spanish specimen (Trappe23377; nLSU sequence: JN37847) were recently generated as part of this study. This specimen was morphologically congruent with the lectotype specimen of the species (a duplicate sent from Muséum national d’Histoire naturelle [PC] to the USDA Forest Service Herbarium, Oregon, USA), and it was conspecific to O. asterosperma specimens collected from UK in the ITS phylogeny (Fig. 4). This indicates that the name O. asterosperma has erroneously been applied to more than one European species. The widespread use of the name O. asterosperma may obscure the true species distribution and confuse the taxonomy of other European Octaviania species, many of which have not been reported or closely studied in recent decades.
Although it is possible to identify most of the Japanese species of Octaviania morphologically, it is difficult or in some cases almost impossible to discriminate between the species in the subg. Parcaea (= Octaviania clade A) based on morphology alone. Accurate species delimitation within this subgenus is only currently possible via DNA sequences. All the phylogenetic analyses except nLSU single-gene analyses were highly informative and clearly separated the four cryptic species of subg. Parcaea (Fig. 1, 2, 3, 4, 5). To evaluate the fidelity of these cryptic species, we also conducted distance-based network analyses, which visualize all the possible topology inferred from a dataset and, thus, can help to identify traces of hybridization, recombination or horizontal gene transfer (Huson 2009, Huson & Bryant 2006). Although we cannot conclude reproductive isolation of each species based only on the few DNA loci used in this study, given the sympatric nature of these species (i.e., geographic distribution and vegetation), we consider that the networks shown in Fig. 4 authenticate that these cryptic species are reproductively independent (e.g., would be recognized by the biological species concept; Mayr 1942). Although the description of morphologically cryptic species is confusing, it is indispensable for precise understanding of speciation, phylogeography, and accurate conservation of species in the subg. Parcaea. This study also shows that phylogenetic network methods can contribute to species-level or infraspecific delimitation of sequestrate fungi, since many of these fungi are so difficult to culture that it is unrealistic to directly apply the biological species concept to their taxonomy.
Phylogeography of Octaviania
Despite the fact that only two taxonomically undetermined Octaviania spp. had previously been reported in Japan (Kobayashi et al. 1987, Yoshimi & Doi 1989) our data indicate that the genus is highly diverse across the country (Fig. 1–4). In the most well resolved phylogeny based on the multigene dataset (Fig. 3), the 12 species-level lineages were scattered across the 3 subgenera (i.e., clades A–C). Octaviania asahimontana is nested within the subclade B, which also includes European, Australian, and North American taxa. Furthermore, this species and O. herperi have independent sister relationships with the North American taxa in subg. Octaviania and Fulvoglobus, respectively (Fig. 1–3). Geographically, the mainland of Japan had repeatedly been connected to the Asian continent until the middle Pleistocene via Tsushima and Tsugaru Straits (c. 0.16 Mya B.P. and 0.13 Mya B.P., respectively; Kizaki & Oshiro 1977, Fujii 1990, Oshima 2000; Map 1). Given that sequestrate fungi depend largely on animal mycophagy for their spore dispersal (Kretzer et al. 2005) and that overseas spore dispersal by these fungi is thought to be rare, the extant species in Japan are considered to have been derived from the continent before the last submergence of these land bridges. From a phylogeographical standpoint, this strongly suggests that Octaviania is also highly diverse in China and Southeast Asia, where the genus has not been adequately sampled. This also implies that records of the East Asian Octaviania species need to be re-evaluated since Asian specimens are usually assigned to European or North American species names (e.g., Tao et al. 1998). Additionally, more extensive examination of the East Asian Octaviania collections will be essential for more precise understanding of biogeographical history of Japanese Octaviania spp. as well as that of Southeast Asian species such as O. borneensis and O. malaiensis.
Within O. nonae, there were slight but distinct differences in the ITS and nLSU sequences between Amami-oshima and Honsyu/Kyusyu (mainland) specimens (i.e., 2.6 % in ITS and 0.9 % in nLSU). This could reflect geographic isolation of the northern Ryukyu superisland including Amami-oshima Island from the continent by the Tokara Strait, which had been formed by the early Pleistocene (Hikida & Ota 1997, Ota 1998, Otsuka & Takahashi 2000; Map 1). The Tokara Strait is biogeographically considered to be the border between Palearctic and Oriental regions, and similar divergence is widely recognized in amphibians and reptiles between these regions (Hikida & Ota 1997, Toda et al. 1999). This strongly suggests that the four cryptic species of subg. Parcaea had speciated by the Gelasian in the early Pleistocene (1.806–2.588 Mya B.P.), the era that the Japanese Archipelago had been connected to the Asian continent. Based on this estimation, subg. Parcaea is considered to be of continental origin and it is highly possible that other unknown species of the subgenus are present in East or Southeast Asia. As far as we know, this is the first example of distinct phylogeographic divergence of sequestrate fungi between the mainland of Japan and the Ryukyu Archipelago.
Another phylogeographically notable relationship is that the O. etchuensis specimen collected from Toyama Prefecture, central Honsyu (the mainland of Japan), was sister to O. yaeyamaensis collected from Ishigaki Island, one of the southernmost islands of Japan and c. 1 800 km distant from Toyama Prefecture (Map 1). This seems strange based on the present geography of Japan, but can be explained in accordance with paleogeography of the region. The Ishigaki Island had been repeatedly connected to Taiwan and China via a land bridge until the middle or late Pleistocene (Ota 1998, Kimura 2000). Thus, we presume that the common ancestor between O. etchuensis and O. yaeyamaensis was present on the Asian continent and it had already diverged from the other lineages of the clade C shown in Fig. 3. This also implies that extant species closely related to O. etchuensis and O. yaeyamaensis might still be present in the region.
Acknowledgments
We are grateful to Michael A. Castellano, Rosanne Healy, Tsuyoshi Hosoya, Teresa Lebel, James M. Trappe, J.W. Spatafora, Alija Mujic and Roy Halling for facilitating access to herbarium specimens. Shuichi Niida provided us the map of Japan used for Map 1. We also thank Yoko Ando, Ikuo Asai, Fumiko Deai, Atsuko Hadano, Eiji Hadano, Makoto Hashiya, Shunsuke Hirose, Sumio Honda, Toshikazu Imoto, Akiko Kajiyama, Naoki Kajiyama, Jun-ichi Kikuchi, Taiga Kasuya, Jun-ichi Kikuchi, Yusei Kitade, Sin-ichi Kudo, Masanori Kutsuna, Yuzou Kotera, Shinya Kohtoku, Hideko Miwa, Tetsuo Muroi, Michiyo Nabe, Mitsuo Nabe, Minoru Nakajima, Fumitaka Nagao, Eiji Nagasawa, Masaru Ohkubo, Muneyuki Ohmae, Ayako Okuda, Haruko Saiki, Hiromi Sasaki, Seikichi Sato, Yo-ichi Sunada, Toshiho Ueda for providing valuable specimens or supporting the field work of the first author. Members of the volunteer mycology group at KPM kindly assisted in the accessioning of herbarium specimens for this study. The study was supported by Research Fellowships for Young Scientists (No. 21-6052) from the Japan Society for the Promotion of Science (JSPS) and partially by the Grant-in-Aid for the Global COE Program ‘Advanced Utilization of Fungus/Mushroom Resources for Sustainable Society in Harmony with Nature’ from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
REFERENCES
- Binder M, Hibbett DS. 2006. Molecular systematics and biological diversification of Boletales. Mycologia 98: 971–981 [DOI] [PubMed] [Google Scholar]
- Cázares E, Garcia J, Castillo J, Trappe JM. 1992. Hypogeous fungi from northern Mexico. Mycologia 84: 341–359 [Google Scholar]
- Chu-Chou M, Grace LJ. 1983. Hypogeous fungi with some forest trees in New Zealand. New Zealand Journal of Botany 21: 183–190 [Google Scholar]
- Coker WCR, Couch JN. 1928. The gasteromyces of the eastern United States and Canada. Waverly Press, Baltimore, Maryland, USA [Google Scholar]
- Corner HJH, Hawker LE. 1953. Hypogeous fungi from Malaya. Transactions of the British Mycological Society 36: 125–137 [Google Scholar]
- Cribb JW. 1958. The Gasteromycetes of Queensland-V. Octaviania and Hydnangium. Papers from the Department Botany, University of Queensland 3: 245–254 [Google Scholar]
- Cunningham GH. 1944. The Gasteromycetes of Australia and New Zealand. Reprint by Bibliotheca Mycologica Bd. 67, Cramer, Germany. [Google Scholar]
- Desjardin DE, Wilson AW, Binder M. 2008. Durianella, a new gasteroid genus of boletes from Malaysia. Mycologia 100: 956–961 [DOI] [PubMed] [Google Scholar]
- Dodge CW, Zeller SM. 1936. Hydnangium and related genera. Annals of the Missouri Botanical Garden 3: 565–598 [Google Scholar]
- Farris JS, Källersjö M, Kluge AG, Bult C . 1995. Testing significance of incongruence. Cladistics 10: 315–319 [Google Scholar]
- Frank JL, Barry S, Southworth D. 2006. Mammal mycophagy and dispersal of mycorrhizal inoculum in Oregon white oak woodlands. Northwest Science 80: 264–273 [Google Scholar]
- Fujii S. 1990. Changes of the Palaeoenvironment along the Japan Sea since the Early Pleistocene. The Quaternary Research 29: 173–182 [In Japanese with English abstract.] [Google Scholar]
- Galtier N, Gouy M, Gautier C. 1996. SEAVIEW and PHYLO_WIN: Two graphic tools for sequence alignment and molecular phylogeny. Computer Applications in the Biosciences 12: 543–548 [DOI] [PubMed] [Google Scholar]
- Gams W. 1999. Proposals to conserve or reject. Report of the Committee for Fungi: 8. Taxon 48: 807–810 [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118 [DOI] [PubMed] [Google Scholar]
- Geyer CJ. 1991. Markov chain Monte Carlo maximum likelihood. In: Keramidas EM (eds), Computing science and statistics: Proceedings of the 23rd symposium on the interface: 156–163. Fairfax Station, Interface Foundation, Virginia, USA. [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 96/98/NT. Nucleic Acids Symposium Series 41: 95–98 [Google Scholar]
- Hawker LE. 1954. British hypogeous fungi. Philosophical transactions of the Royal Society of London. Series B. 237: 429–546 [Google Scholar]
- Hikida T, Ota H. 1997. Biogeography of reptiles in the subtropical East Asian Islands. Proceedings of the Symposium on the Phylogeny, Biogeography and Conservation of Fauna and Flora of East Asian Region: 11–18. National Taiwan Normal University, Taipei, Taiwan, China [Google Scholar]
- Huson DH . 2009Drawing rooted phylogenetic networks. IEEE/ACM Transactions on Computational Biology and Bioinformatics 6: 103–109 [DOI] [PubMed] [Google Scholar]
- Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23: 254–267 [DOI] [PubMed] [Google Scholar]
- Huson DH, Rupp R. 2008. Summarizing multiple gene trees using cluster networks. In: Crandall KA, Lagergren J. (eds), Algorithms in bioinformatics, lecture notes in computer science, 2008, 5251/2008: 296–305. Springer Verlag, Berlin, Germany [Google Scholar]
- Kimura M. 2000. Paleogeography of the Ryukyu Islands. Tropics 10: 5–24 [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. 2008. Dictionary of the Fungi. 10th ed. CAB International, Wallingford, UK [Google Scholar]
- Kizaki K, Oshiro I. 1977. Paleogeography of the Ryukyu Islands. Marine Sciences Monthly 9: 542–549 [In Japanese with English abstract.] [Google Scholar]
- Kobayasi Y. 1937. Development and structure of a new species of Octaviania (Hymenogastraceae). The Botanical Magazine, Tokyo 51: 291–299 [Google Scholar]
- Kobayasi Y, Otani Y, Hagiwara H. 1987. Fungal enumeration by Kumagusu Minakata Vol. 1. Sanko Publ. Co., Ltd., Tokyo, Japan [Google Scholar]
- Kretzer AM, Dunham S, Molina R, Spatafora JW. 2005. Patterns of vegetative growth and gene flow in Rhizopogon vinicolour and R. vesiculosus (Boletales, Basidiomycota). Molecular Ecology 14: 2259–2268 [DOI] [PubMed] [Google Scholar]
- Lenne M. 2005. Octaviania asterosperma, un champignon hypogé retrouvé au Bois de la Cambre. Revue du Cercle Mycolgie de Bruxelles 5: 55–64 [Google Scholar]
- Lloyd CG. 1922. Mycological notes no. 67. Mycological writings 7, 2: 1137– 1168 [Google Scholar]
- Maekawa N. 1987. A new species of the genus Ceriomyces. Canadian Journal of Botany 65: 583–588 [Google Scholar]
- Massee G. 1889. A monograph of British Gastromycetes. Annals of Botany 4: 1–103 [Google Scholar]
- Mayr E. 1942. Systematics and the Origin of Species. Columbia University Press, New York, USA [Google Scholar]
- Montecchi A, Sarasini M. 2000. Funghi Ipogei d’Europa. A.M.B. Fondazione Centro Studi Micologici, Trento, Italy [Google Scholar]
- Moreno G, Galan R, Montecchi A. 1991. Hypogeous fungi from Peninsular Spain II. Mycotaxon 42: 201–238 [Google Scholar]
- Nylander JAA. 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden [Google Scholar]
- Orihara T, Sawada F, Ikeda S, Yamato M, Tanaka C, Shimomura N, Hashiya M, Iwase K. 2010. Taxonomic reconsideration of a sequestrate fungus, Octaviania columellifera, with the proposal of a new genus, Heliogaster, and its phylogenetic relationships in the Boletales. Mycologia 102: 108–121 [DOI] [PubMed] [Google Scholar]
- Oshima K. 2000. Nihon-retto syuhen no kaikyo-keisei-shi. Kaiyo Monthly 32: 208–213 [In Japanese.] [Google Scholar]
- Ota H. 1998. Geographic patterns of endemism and speciation in amphibians and reptiles of the Ryukyu Archipelago, Japan, with special reference to their paleogeographical implications. Researches on Population Ecology 40: 189–204 [Google Scholar]
- Otsuka H, Takahashi A. 2000. Pleistocene vertebrate faunas in the Ryukyu Islands: Their migration and extinction. Tropics 10: 25–40 [Google Scholar]
- Pegler DN, Young TWK. 1979. The gasteroid Russulales. Transactions of the British Mycological Society 72: 353–388 [Google Scholar]
- Rehner SA, Buckley E. 2005. A Beauveria phylogeny inferred from nuclear ITS and EF-1α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574 [DOI] [PubMed] [Google Scholar]
- Shimomura N, Aimi T, Matsumoto T, Maekawa N, Otani H. 2007. Ultrastructure of developing basidiospores in Rhizopogon roseolus (= R. rubescens). Mycoscience 49: 35–41 [Google Scholar]
- Singer R, Smith AH. 1960. Studies on secotiaceous fungi IX. The astrogaceous series. Memoirs of the Torrey Botanical Club. 21: 1–112 [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690 [DOI] [PubMed] [Google Scholar]
- Tao K, Liu B, Chang M. 1998. Flora fungorum sinicorum vol. 7. Hymenogastrales Melanogastrales et Gautieriales (ed. by Liu B. ). Science Press, Beijing, China: . [In Chinese.] [Google Scholar]
- Thiers HD. 1984. The secotioid syndrome. Mycologia 76: 1–8 [Google Scholar]
- Thompson JD, Gibson TJ, Pleweniak F, Jeanmougin F, Higgins DG. 1997. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda M, Nishida M, Tu M-C, Hikida T, Ota H. 1999. Genetic variation, phylogeny and biogeography of the pit vipers of the genus Trimeresurus sensu lato (Reptilia: Viperidae) in the subtropical East Asian islands. In: Ota H. (ed), Tropical island Herpetofauna – origin, current diversity, and conservation: 249–270. Elsevier, Amsterdam, Netherlands [Google Scholar]
- Trappe JM, Lebel T, Castellano MA. 2002. Nomenclatural revisions in the sequestrate russuloid genera. Mycotaxon 81: 195–214 [Google Scholar]
- Vidal JM. 2004. The genus Stephanospora Pat., two new combinations. Revista catalana de Micologia 26: 97–111 [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. The Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittadini C. 1831. Monographia Tuberacearum. Milano, Mediolani, Italy [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322. Academic Press, San Diego, USA [Google Scholar]
- Yoshimi S, Doi Y. 1989. Japanese gasteromycetes notes (1). Memoirs of the National Science Museum 22: 29–41 [In Japanese.] [Google Scholar]
- Zeller SM. 1948. Notes on certain Gasteromycetes, including two new orders. Mycologia 40: 639–668 [PubMed] [Google Scholar]
- Zeller SM, Dodge CW. 1936. Elasmomyces, Arcangeliella and Macowanites. Annals of the Missouri Botanical Garden 3: 599–638 [Google Scholar]