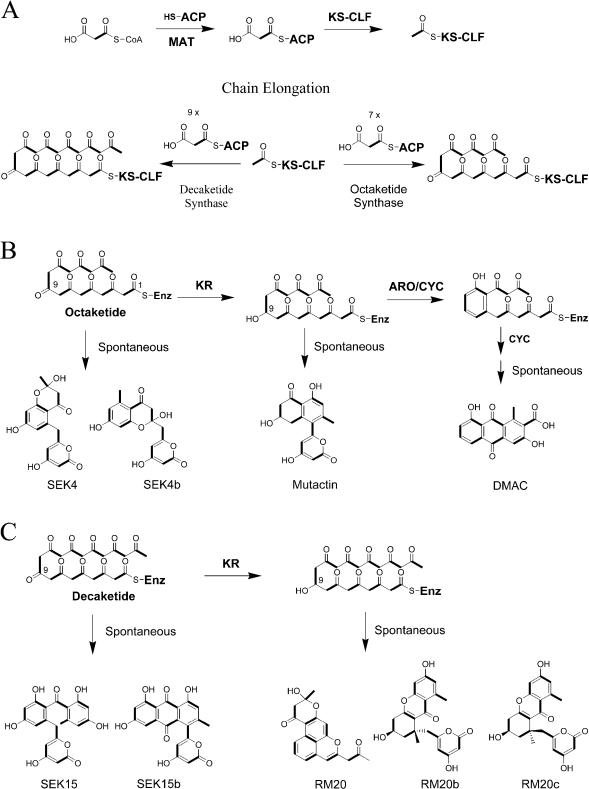
Figure 2. Biosynthesis of Acetate-Primed Polyketides.
(A) Minimal PKS is necessary and sufficient for the synthesis of a complete polyketide chain. KS-CLF is the condensing enzyme in the minimal PKS, catalyzing each round of condensation between malonyl-ACP and the growing polyketide chain. ACP serves as the carrier for malonyl units, and it is malonylated by the MAT associated with FAS. Chain synthesis initiates with the decarboxylation of malonyl-ACP to acetyl-ACP by the KS-CLF for most aromatic PKSs. The acetyl unit is then transferred to the KS-CLF and primes the enzyme for subsequent condensations. The overall chain length is controlled by the KS-CLF complex. An octaketide synthase (e.g., act PKS) uses a total of eight malonyl equivalents (including the primer), while a decaketide synthase (e.g., tcm PKS) uses a total of ten malonyl equivalents.
(B) An octaketide can spontaneously form SEK4 and SEK4b in the absence of tailoring enzymes. Members of the act KR family can regioselectively reduce the octaketide at C-9, which can then spontaneously form mutactin in the absence of AROs and CYCs. When bifunctional ARO/CYC (e.g., actVII) and second-ring CYC (e.g., actIV) are present, the reduced octaketide can be transformed into the anthraquinone DMAC.
(C) A decaketide can spontaneously form SEK15 and SEK15b in the absence of tailoring enzymes. KR can regioselectively reduce the C-9 carbonyl. A reduced decaketide can spontaneously form RM20, RM20b, and RM20c. Not shown are the other tailoring enzymes associated with decaketides, which can transform the nascent decaketide into tetracycline or anthracycline structures.