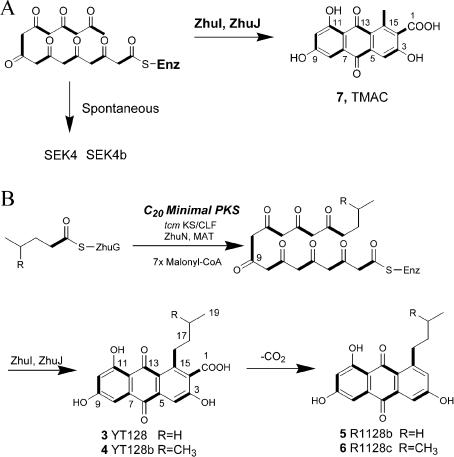
Figure 5. Engineered Biosynthesis of TMAC (7), YT128 (3), and YT128b (4).
(A) ZhuI and ZhuJ are CYCs specific for unreduced octaketides. CH999/pYT105, which coexpressed ZhuI and ZhuJ from the R1128 PKS with the act minimal PKS, produced the anthraquinone compound TMAC (7). ZhuI and ZhuJ are thus able to cyclize unreduced octaketides. Previously characterized octaketide CYCs act ARO/CYC and act CYC are specific for reduced octaketides only. ZhuI and ZhuJ are used for reconstituting R1128 biosynthesis.
(B) Reconstitution of R1128 biosynthesis using the heterologous combination of tcm minimal PKS, R1128 loading module, and CYCs ZhuI and ZhuJ. The alkylacyl-ZhuG intermediate synthesized by the loading module is able to prime the decaketide synthase from tcm minimal PKS. Owing to backbone size restrictions, the tcm KS-CLF primed with the alkylacyl groups are only able to extend the polyketide by seven additional malonyl units, resulting in an alkyl-octaketide. ZhuI and ZhuJ are able to transform the unreduced octaketide into YT128 (3) and YT128b (4). The decarboxylated versions of 3 and 4, which are R1128b (5) and R1128c (6), respectively, are also observed in the extracts of CH999/pYT128.