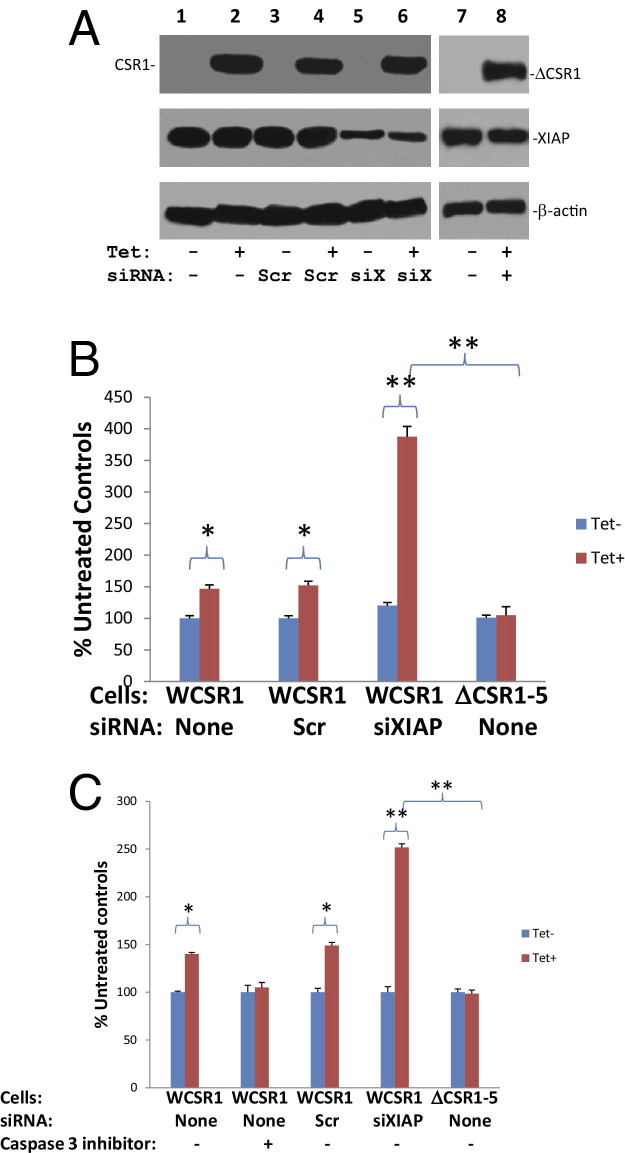
Figure 3.
CSR1 activates the protease activity of caspase-9 and -3. A: Immunoblot analysis of CSR1, XIAP and β-actin. WCSR1 (PC3 transformed with pCDNA4-CSR1/pCDNA6) cells were transfected with siXIAP or scramble control (Scr), and treated with or without tetracycline for 48 hours. The cell lysates were separated by SDS-PAGE and then immunoblotted with the indicated antibodies. Similar tetracycline treatment and assays were performed with ΔCSR1-5 cells (pCDNA4-CSR1Δ513–572/pCDNA6 transformed PC3 cells). B: Activation of caspase-9 by CSR1 is dependent on XIAP. WCSR1 or ΔCSR1-5 cells were transfected with siXIAP or Scr and treated with tetracycline to express wild-type or mutant CSR1, respectively, and cell lysates were incubated with caspase-9 substrate (LEHD-pNA). The protease activity of caspase-9 was then quantified in a spectrophotometer. Triplicate experiments of each treatment were analyzed. Reactions without caspase-9 substrate were used as negative controls. *P < 0.01; **P < 0.001. C: Activation of caspase-3 by CSR1 is dependent on XIAP. WCSR1 or ΔCSR1-5 cells were transfected with siXIAP or Scr, and treated with tetracycline to express wild-type or mutant CSR1, respectively, and cell lysates were incubated with caspase-3 substrate (DEVD-AFC). The protease activity of caspase-3 was then quantified in a spectrofluorometer. Triplicate experiments of each treatment were analyzed. Reactions without caspase-3 substrate were used as negative controls. The specificity of the assay was evaluated using caspase-3 inhibitor. *P < 0.01; **P < 0.001.