Abstract
Tumor microenvironment plays a critical role in tumor initiation and progression. Components in the microenvironment can modulate the growth of tumor cells, their ability to progress and metastasize. A major venue of communication between tumor cells and their microenvironment is through polypeptide growth factors and receptors for these growth factors. This article discusses three major classes of growth-stimulatory polypeptide growth factors and receptors for these growth factors. It also discusses how deregulation of these growth factors or their receptors can drive malignant transformation and progression.
Keywords: Tumor microenvironment, growth factor, EGFR, FGF, PDGF
2. INTRODUCTION
The microenvironment is the environment at the cellular level in which cells interact with each other and with the extracellular matrix (ECM). This interaction is critical in regulating normal epithelial cell growth and differentiation. Extracellular signals play a critical role in tightly regulating the growth and differentiation programs of epithelial cells. Defects in such signalings may circumvent the normal pathway of epithelial differentiation and propels the cells in the direction of malignant transformation. The focus here is on epithelial cells because the overwhelming majority of cancer incidence is of epithelial origin. The microenvironment is extremely complex and consists of components of the ECM, connective tissue stromal cells, and polypeptide growth factors. The ECM itself is composed of complex components of proteoglycans. Major components of the ECM include families of fibronectins, laminins and collgagens. The ECM also consists of other less studied glycoaminoglycans and we do not understand the functional role of these molecules in the microenvironment.
In this microenvironment, epithelial cells not only interact with each other, but also interact with mesenchymal cells and the ECM. These interactions are quite specific. Cell-cell interactions are mediated by specific cell-cell adhesion molecules (1) while cell-matrix interactions are mediated by specific integrin receptors for each of the major components of the ECM (2). It has long been recognized that changes in the microenvironment accompany the transformation process (3). This is often indicated by increased fibroblast proliferation and extensive ECM remodeling in areas where cancer cells are found (4). The tumor stroma in many aspects resembles the processes of wound healing and inflammatory response (5).
The microenvironment is rich in polypeptide growth factors (PGF) and PGFs mediate their action through specific cell-surface receptors. A PGF binds to its cell-surface receptor and initiates intracellular signal cascades that lead to the modulation of gene expression (6). Different PGFs target different cell types. In epithelia, the end-result of PGF action is to exert growth and differentiation control. Both mesenchymal and epithelial cells contribute to the production of PGFs into the microenvironment. Therefore, abnormal production or abnormal cellular responses to PGFs are underly malignant transformation. For example, epidermal growth factor receptor (EGFR) function is frequently deregulated in epithelial tumors, and EGFR signaling has been shown to play an important role both in cancer progression and in epithelial to mesenchymal transition (7). In mammary epithelial cells, constitutively active insulin-like growth factor-1 receptor (IGF-IR) induces cells to undergo epithelial to mesenchymal transition which is associated with a dramatical increase in migration and invasion (8). Moreover, it is believed that tumor epithelial cells and stromal components communicate through the production of growth factors and cytokines (9). For example, tumor cells often release platelet derived growth factor (PDGF), for which stromal cells, notably fibroblasts, myofibroblasts and macrophages, possess receptors; the stromal cells reciprocate by releasing insulin-like growth factor 1 (IGF-1), which benefits the growth and survival of nearby cancer cells (10). Similarly, neoplastic cells within melanomas release PDGF, which elicits IGF-2 production from nearby stromal fibroblasts; this IGF-2 helps to maintain the viability of the melanoma cells (11).
This article reviews three major classes of PGF families in the microenvironment and their cell-surface receptors. We will discusss how these ligand/receptor systems contribute to malignant transformation and progression. These PGFs are the epidermal growth factors, fibroblast growth factors and the platelet-derived growth factors. This article is by no means a comprehensive review of all PGFs in the microenviroement but rather focuses on the the major growth-stimulatory classes of PGF. An important family of PGF, the transforming growth factor β, which can serve as both a tumor suppressor and promoter is discussed elsewhere in this review series.
3. EPIDERMAL GROWTH FACTOR AND EPIDERMAL GROWTH FACTOR RECEPTOR IN TUMOR MICROENVIRONMENT
3.1. Epidermal growth factor and epidermal growth factor receptor
The epidermal growth factor receptor (EGFR) belongs to the ErbB family of receptor tyrosine kinases which includes four members: EGFR, ErbB-2, ErbB-3, and ErbB-4 (12). All these trans-membrane proteins have an extracellular ligand-binding domain, a single hydrophobic transmembrane domain and a cytoplasmic tyrosine kinase-containing domain which is activated after binding with peptide growth factors of the EGF-family of proteins (13), such as EGF, transforming growth factor-α (TGF-α), amphiregulin, heparin-biding EGF, β-cellulin and epiregulin. It is well recognized that the EGFR signaling pathways mediate a wide range of cellular responses such as proliferation, differentiation, migration, and survival upon ligand-binding activation. Moreover, amplified expression of EGFR or its ligands, or both, are found in a majority of human carcinomas (14). EGF-like growth factors can be produced either by the same cells that express EGFRs in an autocrine secretion fashion or by the surrounding cells (including stromal cells) in a paracrine secretion fashion (15). (Fig. 1)
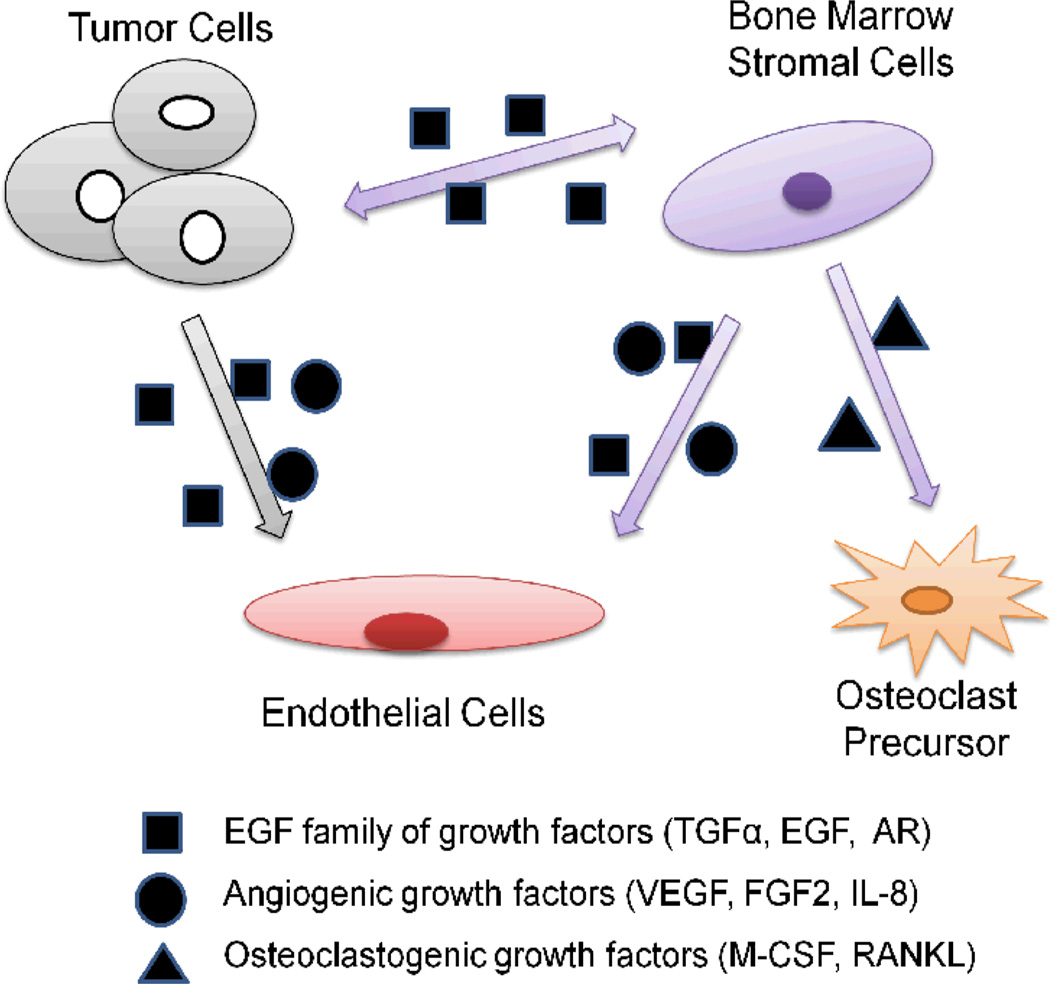
Fig. 1.
Autocrine and paracrine circuits of EGF/EGFR signaling in tumor microenvironment. Cancer cells secrete EGF family of growth factors that can act directly on endothelial cells. Also, EGF family of ligands for EGFR induce the expression of osteoclastogenic factors in bone marrow stromal cells that promote maturation and activation of osteoclasts, leading to bone destruction as well as the formation of bone metastases. In addition, bone marrow stromal cells produce EGF family of growth factors and angiogenic growth factors that can act on both endothelial cells and tumor cells. TGF-α, transforming growth factor α; AR, androgen receptor; VEGF, vascular endothelial growth factor; FGF2, fibroblast growth factor 2; IL-8, Interleukin-8; M-CSF, macrophage colony-stimulating factor; RANKL, receptor activator for nuclear factor κB ligand.
Tumor progression is a complex process that involves interaction of tumor cells with surrounding non-transformed cells and EGFR is expressed in almost all types of stromal cells (12). Therefore, sustained activation of the EGFR signaling in non-malignant cells in the tumor microenvironment might influence the behavior of transformed cells and can play an important role in tumor progression. There is evidence to suggest that overexpression of EGFR protein can be triggered by either gene mutation or by tumor hypoxia which is one of the common features in the tumor microenvironment (16, 17). Meanwhile, the EGFR system is believed to be involved in tumor metastases as well as angiogenesis which are two important phenomena that promote tumor progression (3, 18). Furthermore, a type of small leucine-rich proteoglycan which resides in the tumor microenvironment is capable of downregulating EGFR activity therefore inhibiting both primary tumor growth and metastatic spreading (19).
3.2. Tumor hypoxia and up-regulation of EGFR
It is widely accepted that overexpression of the EGF receptor and amplification of its signal is a common feature in a variety of human cancers including renal, breast, glioma, ovarian, non-small-cell lung, prostate, pancreatic, and head and neck cancers (13). On the other hand, the mechanism underling the amplification of EGFR expression remains poorly understood in human carcinomas. The amplification of the EGFR gene and receptor-activating mutations observed in a few cancers such as glioblastoma multiforme and non-small-cell lung cancer might provide one explanation for the abnormal expression level of EGFR (20, 21). However, these oncogenic phenomena are not common in other tumor types which indicate that the widespread overexpression of EGFR in human cancer might be under the regulation of a more common physiological event in tumors instead of gene amplification and mutations. Among those universal properties of the pathophysiologic tumor microenvironment, hypoxia is the result of the imbalance between the rate of cancer cell proliferation and the ability of the existing vasculature to supply oxygen as a solid tumor grows (22). Importantly, tumor hypoxia, like EGFR expression, is predictive of tumor progression and poor clinical outcome. The correlation between the two has been reported in many studies (17, 23).
3.2.1. The hypoxic tumor microenvironment triggers the expression of EGFR
By using a 3D multicellular tumor spheroid model to mimic the tumor microenvironment (24), Franovic and colleagues have demonstrated that the up-regulation of EGFR protein (but not mRNA) in human cancer cell lines is induced by the hypoxic tumor microenvironment (17). Moreover, they pointed out that tumor hypoxia was not only required but sufficient to up-regulate EGFR protein expression in hypoxic cancer cells. Their findings reveal an important link between tumor hypoxia and up-regulation of the EGFR in the bulk of human cancers that do not display genetic alterations of the receptor. Wang and colleagues performed oligonucleotide microarray analysis to identify the genes associated with the motile phenotype induced by hypoxia in lung adenocarcinoma cells and they found that the expression of EGFR gene was induced more than 5-fold by hypoxia (25). Meanwhile, the immunohistochemical analyses of primary lung adenocarcinomas confirmed the induction of EGFR located in tumor cells in the vicinity of necrotic areas, a histological indicator of tumor hypoxia (26). Overall, these findings proposed an alternative working model by suggesting that tumor hypoxia may represent the common denominator for the aberrant EGFR expression observed in solid tumors.
3.2.2. The role of EGFR in hypoxia-mediated tumor progression
There is now increased evidence showing that hypoxia increases the motility of cancer cells via several pathways thus facilitating the metastasis of cancer cells. In the Wang and colleagues’ study, they showed that hypoxia inhibited cell-cell adhesion and increased migratory ability in lung adenocarcinoma cells (25). In addition, the increased motility was blocked by an inhibitor of EGFR. Thus, these authors have demonstrated the importance of the EGFR pathway in the induction of motility of cancer cells in a hypoxic tumor environment.
3.3. Role of EGFR in the Pathogenesis of Metastasis
The metastatic potential of tumor cells is believed to be regulated by interactions between tumor cells and their surrounding environment (ECM and stromal cells) (27). The development of metastasis is complex, requiring multiple distinct steps to successfully establish a tumor at a secondary site (28). For dissemination and metastasis to occur, tumor cells must invade the tissue surrounding the primary tumor, intravasate into the lymphatic system or blood supply system, extravasate from the vascular system into a secondary organ and initiate angiogenesis in order to enable proliferation at that site (29). (Fig. 2)
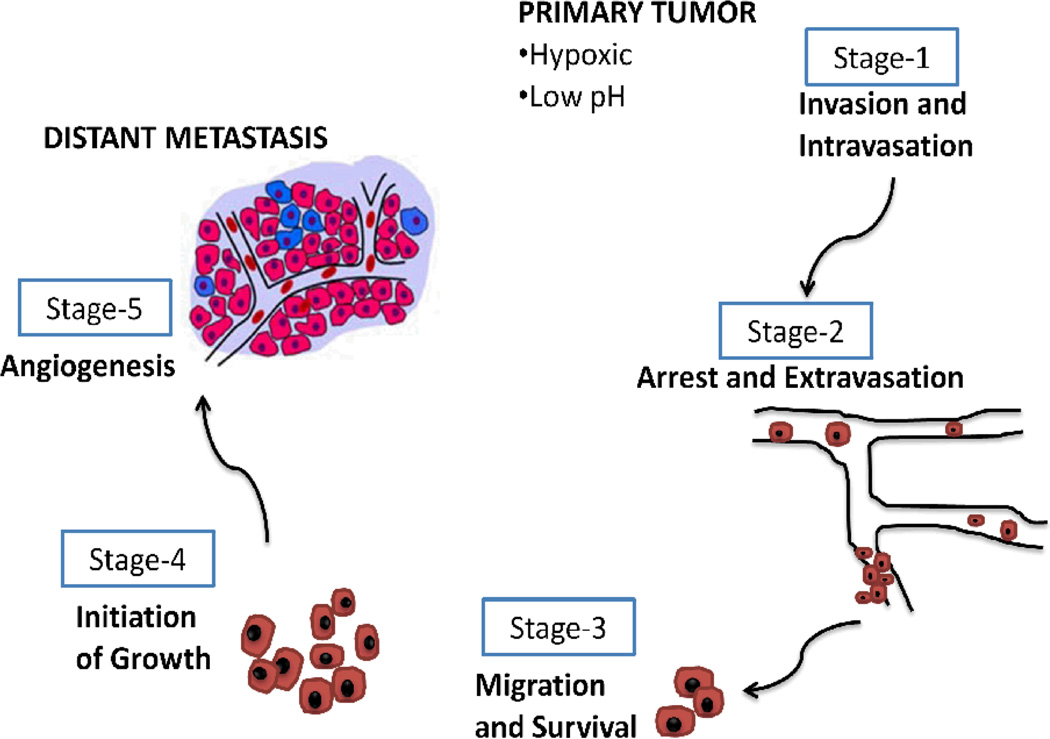
Fig. 2.
Tumor metastatic process. The metastatic process can be broken down into 5 stages. After breaching basement membrane, the primary invasive tumor cells may intravasate into either lymphatic or blood microvessels. The blood can then transport these cancer cells to distant anatomical sites, where they may be trapped and subsequently extravasate and form dormant micrometastases. Eventually some of the micrometastases may acquire the ability to colonize the tissue in which they have landed, enabling them to form a macroscopic metastasis.
Tissue architecture, integrity and function is intimately connected with cell-cell and cell-extracellular matrix interactions. The metastatic process involves multiple changes at the molecular level that disrupt and modify these interactions (30, 31). These include tissue remodeling through the action of proteinases, such as metalloproteinases (MMPs), apoptotic machinery, as well as chemokines, growth factors and signaling molecules, all of which act together to control processes such as proliferation, survival migration and invasion. Tumor stroma may also facilitate the spread of metastatic cells, as stromal cells derived from lymph nodes can increase the proliferation of tumor cells through the release of an insulin-like growth factor and epidermal growth factor (32).
Sasaki and colleagues have shown that activation of TGF-α-EGFR signaling in colon cancer cells can create a microenvironment that is conducive for metastasis (33). Pu and colleagues have demonstrated that the migration of breast cancer cells directed by an electric field requires ErbB-signaling (34). Wei and colleagues’ work showed that Esophageal squamous cell carcinoma lymph node metastases generally have a high level of EGFR expression in cell membranes similar to that in primary tumors (35). Extensive work has also been done to show that EGFR signaling regulates the ability of bone marrow stromal cells to produce osteoclastogenic factors (specialized progeny of hemopoietic precursors committed to the monocyte/macrophage lineage that, upon certain stimuli, fuse by giving rise to mature bone resorbing cells) (36) and to sustain osteoclast activation (37).
3.3.1. The direct role of EGFR in stimulating osteoclast
Cancer cells are able to synthesize many growth factors and cytokines that lead to the activation of osteoclasts (36). Parathyroid hormone related protein (PTHrP) is believed to be the main mediator of breast cancer-induced bone resorption (38). Zhu and colleagues have reported that EGF-like ligands strongly stimulate osteoclast formation in co-culture of osteoblastic cells with bone marrow macrophages, the precursors for osteoclasts, by regulating the expression of osteoprotegerin, a cytokine which can inhibit the production of osteoclasts, and monocyte chemotatic protein-1 in osteoblastic cells (39). Because co-culture of osteoblastic cells with bone metastatic breast cancer MDA-MB-231 cells had similar effects on the expression of osteoprotegerin and monocyte chemotatic protein-1 in the osteoblastic cells, and those effects could be partially abolished by EGFR inhibitor, the authors concluded that EGF-like ligands, similar to PTHrP secreted by tumors cells, may contribute to osteolytic lesions in bone metastases.
3.3.2. EGFR in mesenchymal stem cells mediated osteoclast differentiation
However, most osteotropic factors do not directly stimulate osteoclast, but rather act indirectly by binding to accessory cells of the bone marrow microenvironment, such as specialized endothelial cells and mesenchymal stem cells (MSC) (40). Since expression of EGF has been demonstrated to occur in osteoclasts (41), it is conceivable that paracrine circuits involving EGFR and its ligands are operating between osteoclasts and osteoblasts. More recently, the functional role of EGFR signaling in MSC has been investigated. Krampera and colleagues reported that activation of EGFR by heparin-binding EGF-like growth factor increased cell proliferation and prevented adipogenic, osteogenic, and chondrogenic differentiation in human bone marrow-derived MSC (42). The ability of conditioned medium from gefitinib-treated MSC-like cells to sustain the differentiation of pre-osteoclasts was significantly reduced as compared with untreated cells reported by Normanno and colleagues (40). These results have demonstrated that the EGFR regulates the ability of MSC to induce osteoclast differentiation. Normanno group’s findings were confirmed by Angelucci and colleagues who demonstrated that treatment with gefitinib significantly reduced the ability of conditioned medium from prostate cancer cells to induce expression of receptor activator for nuclear factor κB ligand in osteoblasts (43). In this regard, it is well established that EGFR and several of its ligands are expressed by prostate cancer cell lines and human primary prostatic carcinomas (44).
3.4. EGFR signaling regulated by tumor microenvironment
Epidermal growth factor receptors can be directly bound by a small leucine-rich porteoglycan (45), decorin, which is primarily synthesized by fibroblasts and myofibroblasts typically located within the tumor microenvironment (46) and affects the biology of different types of cancer by downregulating the activity of several receptors involved in cell growth and survival. Decorin binds to and modulates the signaling of epidermal growth factor receptor and other members of the ErbB family of receptor tyrosine kinases (47). After binding, the receptor dimerizes and is subsequently internalized and degraded in the lysosomes. (Fig. 3) From a physiological point of view, it is relevant to note that decorin can compete with EGF, the EGFR natural ligand, for receptor binding (48). Decorin inhibits tumor cell proliferation by evoking a signaling cascade that is different than the one evoked by EGF, possibly by inducing a different EGFR conformation and selectively activating phosphotyrosines in the receptor autophosphorylation domain (49).
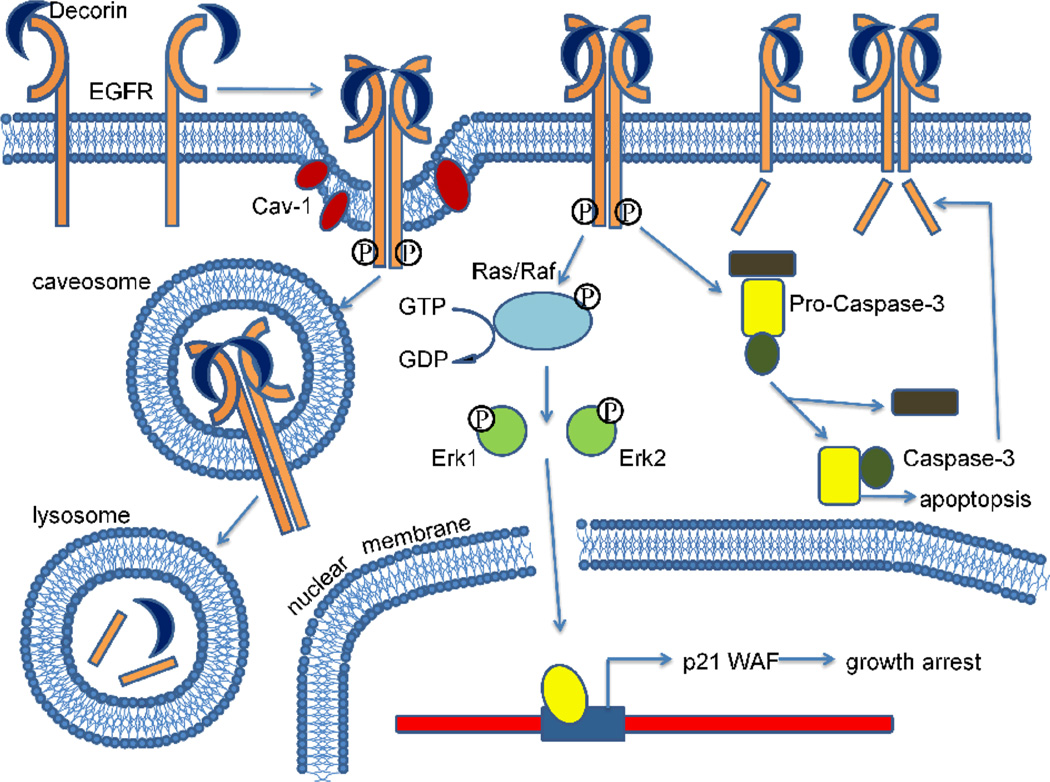
Fig. 3.
Decorin binds to the epidermal growth factor receptor and evokes a unique signaling cascade. Decorin directly binds to the epidermal growth factor receptor and causes its dimerization, internalization and ultimately lysosomal degradation. Upon decorin binding, the epidermal growth factor receptor is phosphorylated, leading to Erk1/2 activation, thus induces the expression of the endogenous cyclin-dependent kinase inhibitor p21WAF and a subsequent arrest of the cells in the G1 phase of the cell cycle. Pro-caspase-3 is cleaved into active caspase-3, which degrades the epidermal growth factor receptor C-terminus and starts the apoptotic process.
Decorin induces apoptosis in a squamous cell carcinoma model via activation of caspase-3 and this effect is dependent on the ability to phosphorylate the EGFR (50). Caspase-3 can cleave the intracellular domain of the EGFR, an additional mechanism by which decorin could downregulate the receptor activity. Decorin also suppresses the activity of ErbB2 and ErbB4 receptors via degradation (26). This effect is most likely achieved indirectly by binding to the EGFR and affecting EGFR/ErbB2 and EGFR/ErbB4 heterodimerization equilibrium.
4. FIBROBLAST GROWTH FACTOR AND RECEPTOR IN TUMOR MICROENVIRONMENT
4.1. Fibroblast growth factor and fibroblast growth factor receptor
The Fibroblast growth factor (FGF) signaling complex comprises one of the twenty-two signaling polypeptides defined by homology, one of a large number of combinatorial splice variants from four genes that encode an FGF receptor (FGFR) transmembrane tyrosine kinase and one or more of a host of poorly characterized, but FGF- and FGFR-specific, structural motifs within heparan sulfate (51). Specificity in FGF signaling lies in the combination of FGFR kinase isotypes, the heparan sulfate that combines with it, and the type of activating FGF polypeptide (52). In addition to endocrine signaling, in which the signal originates outside the tissue expressing the receptor, two types of intra-tissue signaling have been described in reference to the origin of signal, i.e., autocrine and paracrine signaling. FGFs display a broad spectrum of biological function including neurotrophic activity, angiogenic activity, lymphangiogenic effect, stimulation of stem cell differentiation, osteogenesis, tumor cell migration and invasion, and mediating stromal-epithelial cell cross-talk (53). Chesi and colleagues found that FGFR3, when overexpressed in multiple myeloma, may be not only oncogenic when stimulated by FGF ligands in the bone morrow microenvironment but is also a target for activating mutations that enable FGFR3 to play a ras-like role in tumor progression (54). Nomura and colleagues have shown that stromal FGF10 induces migration and invasion in pancreatic cancer cells through interaction with FGFR2, resulting in a poor prognosis (52).
4.2. Fibroblast growth factor-mediated stromal-epithelial cross talk in prostate tumors
Reciprocal communication between stromal and epithelial compartments underlies normal development and homeostasis in the adult prostate gland (55). The emergence of autonomous epithelial cells independent of microenvironmental restraints imposed by prostate stroma or distal sites of metastasis is a hallmark of malignant carcinoma (56, 57). In the prostate and several other parenchymal organs with distinct epithelial and stromal compartments, expression of FGF7 and FGF10 is limited to stromal cells (58). A specific FGFR complex of heparan sulfate and splice variant FGFR2IIIb that recognizes FGF7 and FGF10 is expressed only in epithelial cells (59). Stromal to epithelial cell signaling via FGF7/FGF10 and FGFR2IIIb has a net effect of promoting epithelial cell homeostasis that includes growth but limits population growth overall by feedback inhibition mechanisms and induction of differentiation (60).
To study the changes in mutual communication mediated by FGF signaling system between stromal and epithelial cells during malignant progression, Wu and colleagues used a rare rat prostate adenocarcinosarcoma (Dunning R3327PAP) in which stromal and epithelial compartments have evolved as a single mutually interdependent nonmalignant transplantable unit (53). By performing clonal analysis of stromal cells derived from the Dunning R3327PAP adenocarcinosarcoma, and characterizing them according to cytoskeletal markers and expression of signaling polypeptides and receptor isotypes within the FGF family, they found two distinct subtypes. One had an undifferentiated fibroblast-like character, whereas the other exhibited smooth muscle cell-like properties. The two morphological subtypes exhibited distinct expression patterns with respect to FGF7, FGF10, FGFR1, FGFR2IIIc, and FGFR3. Their study suggested a specific epithelial to stromal cell part of a two-way stromal-epithelial cell dialogue in premalignant slow-growing differentiated tumors of which the disruption may contribute to the progression of epithelial cells to malignancy (61).
Jin and colleagues found FGF9 was expressed significantly only in epithelial cells and that it binds only to FGFR3 that is present only at functionally significant levels in stromal cells in a two-compartment transplantable prostate tumor model in which survival of stromal cells in vivo depends on epithelial cells (61). Their data indicated that the FGF9/FGFR3 pair completes a two-way paracrine communication circuit between prostate epithelium and stroma that may be important for intercompartmental homeostasis in normal nonmalignant two-compartment tumors and subject to subversion during progression to malignancy. (Fig. 4)
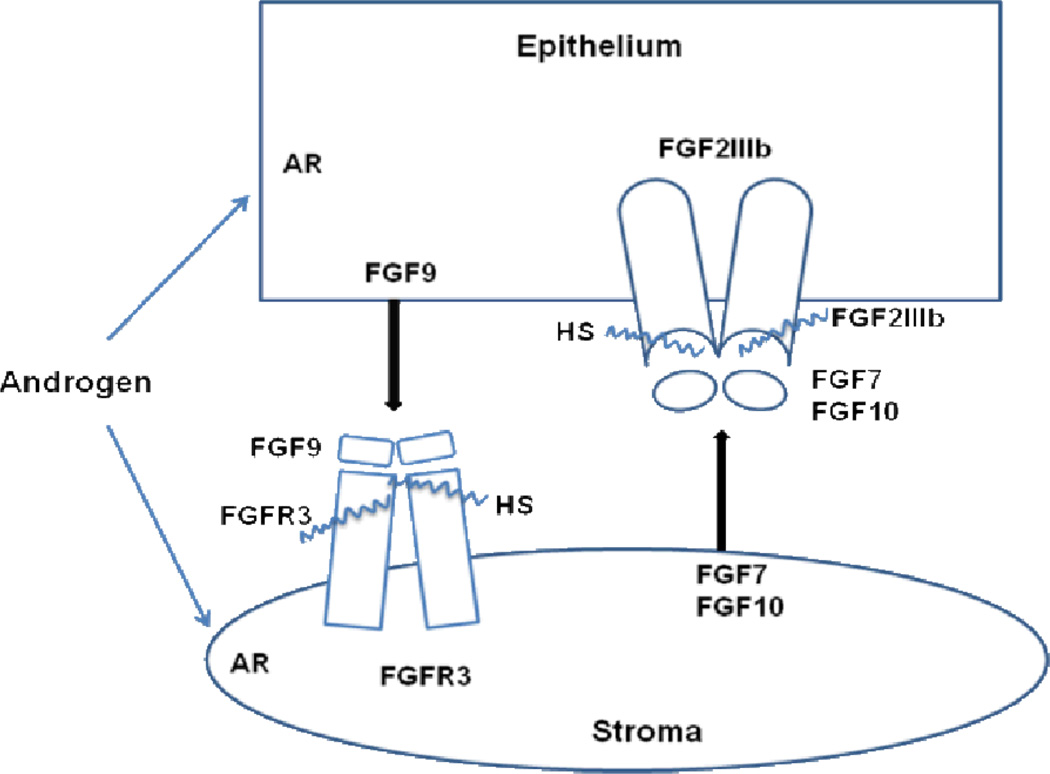
Fig. 4.
Two-way directional communication between stroma and epithelium mediated by fibroblast growth factors. Stromal FGF7/FGF10 and epithelial FGFR2IIIb mediate stromal to epithelial signaling, while epithelial FGF9 signaling to stromal FGFR3 completes the two-way conversation. Androgen may differentially modify either the signal or reception in either compartment. Either partitioned FGFR signal may affect reciprocal cross-talk back to the other compartment. FGF, fibroblast growth factor; AR, androgen receptor; HS, cell- and FGFR-specific heparan sulfate (wavy line).
4.3. Role of fibroblast growth factor in angiogenesis
Tumor angiogenesis, a process of new vasculature formation, is appreciated to be an integral part of solid tumor development (62). The supply of new blood vessels in tumors not only fosters autonomous tumor growth but also helps remove accumulated waste and ameliorate burdensome metabolism. Several experimental evidences point to different classes of FGF receptors has been identified (63). In a fast growing malignant tissue, tumor blood vessels are exposed to multiple growth factors and cytokines (64). Although the role of individual factors and their signaling pathways in regulating tumor neovascularization is relatively well studied, recent research has focused on characterization of interactions among multiple membrane-bound receptors. This focus has lead to the establishment of a paradigm that, instead of transmitting signals across the membrane individually, each membrane-anchored receptor usually associates and coordinates with other adjacent membrane-bound receptors to synergistically induce an array of intracellular signaling cascades (65).
4.3.1. Angiogenic synergism between fibroblast growth factor-2 and platelet derived growth factor-BB
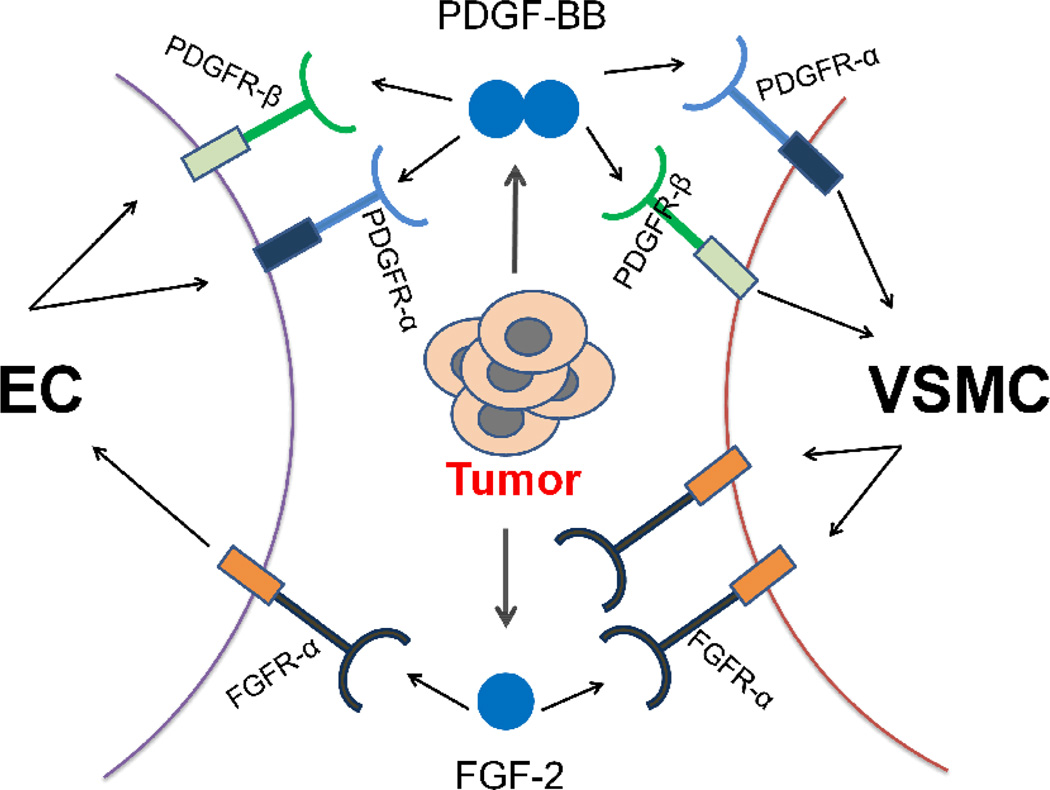
Fibroblast growth factor-2 (FGF-2) is a frequently expressed non-vascular endothelial growth factor (VEGF) angiogenic factor in tumors. For example, high levels of FGF-2 are often present in patients with highly vascularized and advanced cancers (56). Additionally, activated endothelial cells in growing blood vessels are also important sources for platelet derived growth factor-BB (PDGF-BB) and a malignant transition of tumor cells could lead to active secretion of FGF-2 from tumor cells (62, 66). Thus, FGF-2 and PDGF-BB are often co-expressed in the same tumor tissue (67). As endothelial cells usually express an undetectable level of PDGF receptors (PDGFRs), angiogenic synergy between PDGF-BB and FGF-2 suggests that FGF-2 might modulate the PDGF signaling system in endothelial cells. Indeed, high levels of both PDGFR-α and PDGFR-β are only detected in FGF-2-induced angiogenic vessels, but not in VEGF- or PDGF-BB-induced microvessels (67), suggesting that FGF-2 upregulates PDGFR expression in endothelial cells. Also, the FGF-2-induced signaling pathways activate the promoter activity of both PDGFR-α and PDGFR-β in capillary endothelial cells (68). In agreement with increased levels of PDGFR mRNAs, Cao’s group further confirmed that the protein levels of PDGFRs are also dramatically upregulated in endothelial cells. Notably, the FGF-2-pretreated endothelial cells become highly responsive to PDGF-BB-induced motility. Interestingly, PDGF-BB is also able to induce FGFR-1promoter activity in vascular smooth muscle cells, which become more sensitive to FGF-2 stimulation (67). (Fig. 5) Another possible mechanism could be that tumor-derived PDGF-BB disassociates mural cells from the tumor vessels, which become more accessible and sensitive to FGF-2 stimulation. However, if this is one of the mechanisms, PDGF-BB and VEGF would also produce a synergistic effect on angiogenesis. Obviously, a combination of PDGF-BB and VEGF lacks a synergistic activity (69). Thus, the angiogenic synergism between FGF-2 and PDGF-BB involves specifically reciprocal interplay between their receptor signaling systems in endothelial cells and vascular smooth muscle cells.
Fig. 5.
Reciprocal interplay between tumor-derived fibroblast growth factor (FGF) and platelet derived growth factor (PDGF) in promoting tumor angiogenesis. Activation of FGFRs in endothelial cells by tumor-derived FGF-2 leads to switching on the expression of PDGFR-α and PDGFR-β, which acquire responses to PDGF ligand stimulation. PDGF-BB increases sensitization of vascular smooth muscle cells to FGF-2 stimulation by upregulating FGFR-1 expression in these cells. The functional consequence of this reciprocal interaction on the tumor vasculature is manifested by accelerating angiogenic vessel growth. EC, endothelial cell; VSMC, vascular smooth muscle cell.
4.3.2. Angiogenic synergism between fibroblast growth factor-2 and vascular endothelial growth factor
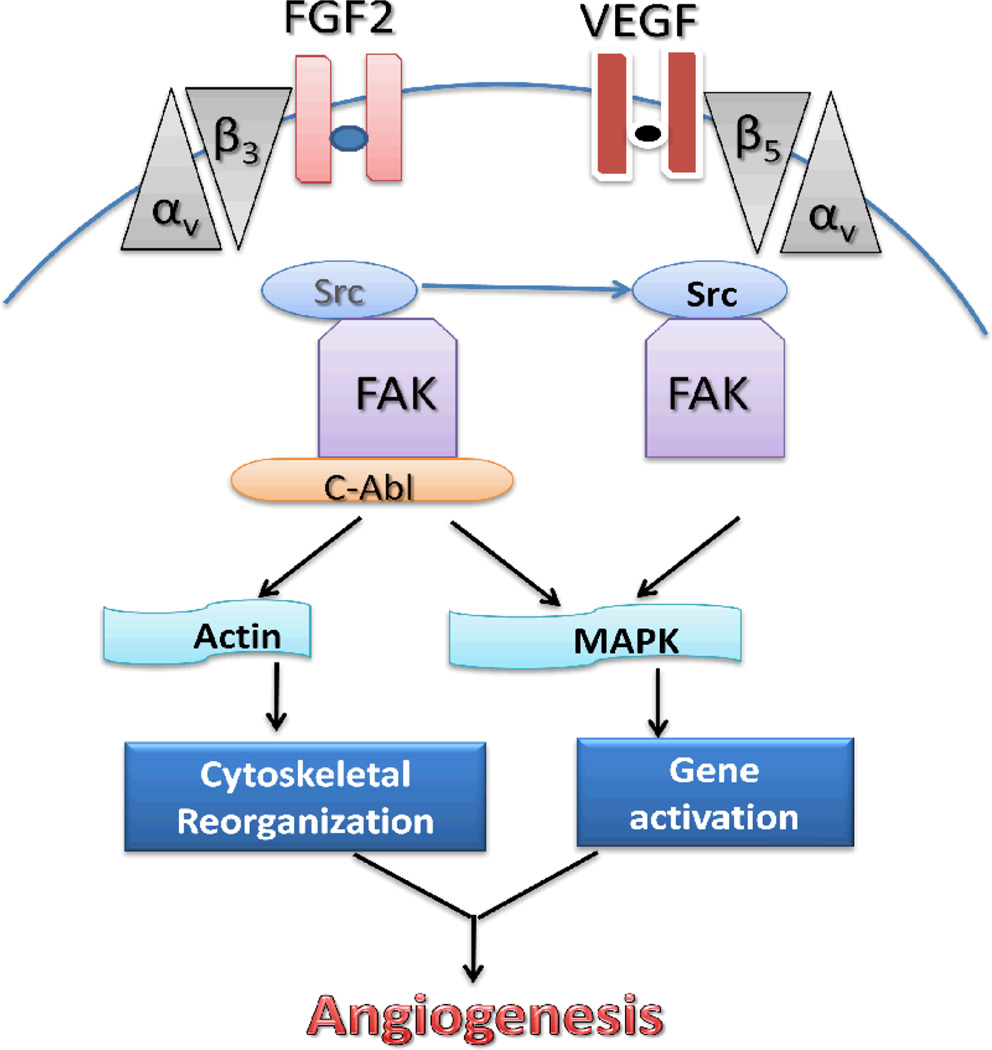
Indraccolo and colleagues suggested that short-term treatment with antiogenic factors FGF-2 or VEGF, either given as recombinant factors or delivered by retroviral vectors, accelerated tumor growth (70). Wei and colleagues showed that FGF-2 and/or VEGF induced angiogenesis can be mediated by a proto-oncoprotein c-Abl, a family member of nonreceptor tyrosine kinases (69). Once endothelial cells are stimulated with FGF-2, the cooperation of membrane-anchored receptors between integrin αvβ3 and FGFR leads to the “out-side in” signaling activation. Then c-Abl disassociates from Src (a family of proto-oncogenic tyrosine kinase) that is connected to integrin αvβ3 and increases in association with activated focal adhesion kinase (FAK), resulting in downstream mitogen-activated protein kinase (MAPK) activation. In addition, c-Abl may directly regulate reorganization of cytoskeleton actin to facilitate cell motility. VEGF engages similar but c-Abl–independent angiogenic machinery in which Src acts as an upstream effector of focal adhesion kinase (69). (Fig.6)
Fig. 6.
A hypothetical model for the signaling pathways induced by fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor (VEGF). When endothelial cells are stimulated with FGF2, the cooperation of membrane-anchored receptors between integrin αvβ3 and FGFR leads to the “out-side in” signaling activation. C-Abl disassociates from Src that is connected to integrin β3 and increases the association with activated FAK. VEGF involves similar, but c-Abl-independent angiogenic machinery in which Src acts as an upstream effector of FAK. FAK, focal adhesion kinase; MAPK, mitogen-activated protein kinase.
4.3.3. Clinical anti- angiogenesis target
Since angiogenesis plays a key role in tumor growth and metastasis, the identification of anti-angiogenic drugs and of angiogenesis-related targets may have significant implications for the development of anti-neoplastic therapies. The teleost zebrafish represent a promising alternative model in cancer research (71). Using this model, Nicoli and colleague developed a zebrafish yolk membrane (ZFYM) angiogenesis assay based on the injection of human recombinant FGF2 in the perivitelline space in the proximity of developing subintestinal vein vessels. Injected rFGF2 induced a rapid and potent angiogenic response reflected in the ectopic growth of newly formed blood vessels. FGF2 antagonist long-pentraxin 3 inhibits the angiogenic activity of rFGF2 when added to fish water or when co-injected with the growth factor, respectively (65). The fibroblast growth factor receptor 3 inhibitor is a novel anti-myeloma agent, which is deregulated as a result of the t (4;14) chromosomal translocation that occurs in approximately 15% of multiple myeloma patients. A highly specific anti-FGF-R3-neutralizing antibody PRO-001, for example, can induce apoptosis in primary t(4;14) multiple myeloma samples (72).
5. PLATELET-DERIVED GROWTH FACTOR AND RECEPTOR IN TUMOR MICROENVIRONMENT
5.1. Platelet-derived growth factor and platelet-derived growth factor receptor
Platelet-derived growth factor (PDGF) is a potent mitogen and chemoattractant for mesenchymal cells, such as fibroblasts, and plays a critical role in wound healing and tumor development. The PDGF family consists of four members, all of which act as a homo or hetero dimmer PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC, and PDGF-DD, which exert their action via binding to two receptor tyrosine kinases, PDGF α-receptors (PDGFR-α) and β-receptors (PDGFR-β) (73). The PDGFR-α binds all isoforms except PDGF-DD, whereas the PDGFR-β only binds PDGF-BB and PDGF-DD with high affinity (74). PDGFR-α plays an important role during early embryonic development and organogenesis. PDGFR-β is widely expressed by mesenchymal cells and is found up-regulated in the granulation tissue during wound healing and chronic inflammation.
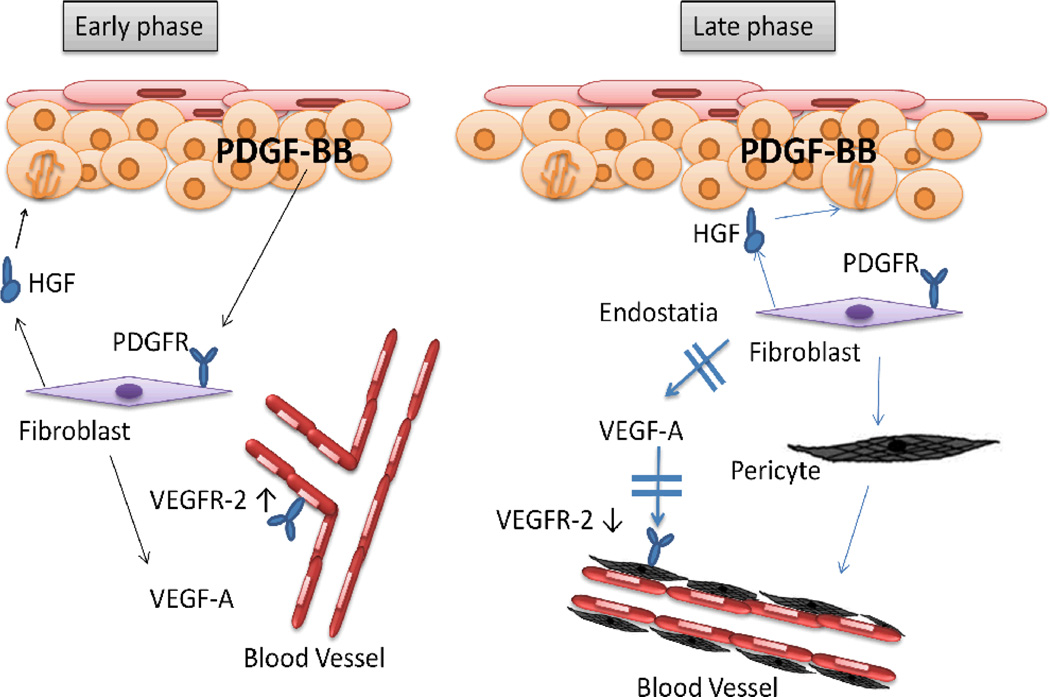
It is widely accepted that tumor cells strongly depend on a reactive stroma, with activated stromal cells playing an important role in tumor growth, invasion, and metastasis (75). Carcinoma-associated or phenotypically altered stromal cells have been demonstrated to promote tumorigenic conversion of preneoplastic cells. In contrast, normal stromal cells were shown to inhibit the growth of carcinoma cells (76). Although growth factors are known to tightly control this complex interplay, the molecular mechanisms underlying these regulatory interactions between the stromal and tumor compartment remains poorly understood. PDGF stimulates tumor growth and progression by affecting tumor and stromal cells (73). With the immortalized human skin keratinocyte cell line HaCat, Lederle and colleague identified fibroblasts as PDGF target cells that are essential for mediating transient angiogenesis and persistent epithelial hyperproliferation based on the fact that in HaCaT/PDGF-B transplants in vivo, the initially enhanced VEGF protein expression by stromal fibroblasts was subsequently reduced coinciding with enhanced pericyte recruitment (77). (Fig. 7)
Fig. 7.
Model for dual time-dependent effects of PDGF-BB. PDGF-BB exerts dual time-dependent effects on stromal fibroblasts that provide a possible explanation for the benign tumor phenotype. In the early phase (left), PDGF-BB secreted by the epithelium activates PDGFR-positive fibroblasts, leading to the up-regulation of VEGF-A and Hepatocyte growth factor (HGF), which is a potent stimulatory factor for epithelial cells and promotes epithelial proliferation. VEGF-A in turn activates the dermal endothelial cells devoid of PDGFR, up-regulates their VEGFR expression, and induces angiogenesis, possibly also supported by the action of HGF. During late phase (right), prolonged exposure to PDGF-BB down-regulates VEGF secretion by dermal fibroblasts, an effect that could be mediated by endostatin (an angiogenesis inhibitor) up-regulation, induces their differentiation into myofibroblasts. Blood vessel maturation is accompanied by VEGFR down-regulation. At the same time, the strong and ongoing increase in HGF secretion continues to stimulate the proliferation of the epithelial cells.
5.2. Role of Platelet-derived growth factor in pancreatic tumor stroma
The predominant mesenchymal cells within the pancreatic cancer stroma is believed to be the stellate cell (78), which is also found in the liver. Most investigators agree that stellate cells are similar to myofibroblasts found in other tumor stroma including breast and prostate cancer (79). The stellate cells play an important role in the microenvironment by mediating fibrosis, facilitating growth and invasion of pancreatic cancer cells (80). Conditioned media from pancreatic cancer cell lines stimulate pancreatic stellate cell activation; this effect is abrogated when PDGF activity is blocked by the use of neutralizing antibodies (81). In other studies, PDGF has been shown to increase pancreatic stellate cell activation and production of ECM proteins (77). Thus, PDGF is likely an important mediator of pancreatic tumor-stromal cell interaction.
Marya and colleagues have suggested that overexpression of PDGF-BB in colorectal cancer and pancreatic cancer cells can result in an increased pericyte coverage of endothelial cells in vivo, rendering the tumor vasculature more resistant to antiangiogenic therapy (82). Pericytes regulate vascular function, including vessel diameter (and thus blood flow) and vascular permeability as well as provide mechanical support and stability to the vessel wall and maintain endothelial cell survival through direct cell-cell contact and paracrine circuits (66, 83). When they stably transfected the cDNA for the PDGF-B into HT-29 human colorectal cancer and FG human pancreatic cancer cells and injected them into mice, an increase in pericyte coverage of endothelial cells in the PDGF-BB–overexpressing tumors together with an inhibition of tumor growth were observed (82). Therefore, increasing the pericyte content of the tumor microenvironment inhibits the growth of angiogenesis-dependent tumors under the regulation of PDGF signaling.
5.3. Role of Platelet-derived growth factor in cervical carcinoma
The stromal compartment is prominent in cervical carcinoma, and recent studies have identified numerous changes in the gene expression pattern of stromal cells in malignant cervical tissue compared to nonmalignant tissue (84, 85). By using a genetically engineered mouse model of cervical carcinogenesis, Pietras and colleagues investigated on PDGFR signaling in cancer-associated fibroblasts and pericytes (86). They found that a pharmacological blockade of PDGF receptor signaling with the clinically approved kinase inhibitor imatinib slowed progression of premalignant lesions. Their subsequent studies indicated that PDGF ligands expressed by cancerous epithelia evidently stimulated PDGFR-expressing stroma to up-regulate FGFs, promoting angiogenesis and epithelial proliferation (86).
5.4. Platelet-derived growth factor in tumor cell induced ‘stromal resistance’
It is widely accept that stromal cells, particularly fibroblasts, support invasive cancer cells of the surrounding tissue for access to the vascular system via paracrine mechanisms (87). Werth and colleagues have provided a novel theory about tumor cells induced ‘stromal resistance’ (protect the microenvironment from oxidative damage) by PDGF regulated pathway (88). They added the supernatant of cultured skin-derived tumor cells to fibroblasts and found the fibroblasts were protected from hydrogen peroxide-mediated cell toxicity. The platelet-derived growth factor secreted from the cancer cells was identified as a trigger of this protection in fibroblasts via the phosphoinositide 3-kinase pathway.
6. PERSPECTIVE
An important venue of communication and interaction between epithelia and the microenvironment is through polypeptide growth factors and cell-surface receptors for these growth factors. This venue of communication is an important mechanism in regulating the proliferation and differentiation of epithelial cells and in maintaining the integrity of epithelia. It is well accepted that disruption of growth and differentiation control mechanisms underlies malignant transformation and progression. In this article, we discussed three major classes of growth stimulatory factors and emphasized on how deregulated expression or function of these ligand/receptor systems can drive malignant transformation. An understanding of growth and differentiation control mechanisms has significant bearing on the chemoprevention and therapeutic intervention of malignant diseases. Agents that can restore normal growth control to malignant cells and restore cell-cell and cell-matrix communication have chemopreventive potential. The retinoids and the relatively new compounds such as the rexinoids and resveratrol exemplify these. Recently, the IGF-1 axis has emerged as a chemopreventive target. The rationale being most cancer cells utilize this axis to sustain proliferation and because IGF-1 is not of physiologic significance in an adult, the development of strategies to down modulate the production of IGF-1 could prevent or delay the carcinogenesis process. In the therapeutic arena, the use of small molecules to block a specific growth factor pathway has led to improvement in therapeutic outcome. This is exemplified by the use of Gefitinib and Erlotinib to disrupt the function of EGF receptor tyrosine kinases in the treatment of non-small cell lung carcinoma. Her2/Neu (a member of the EGF receptor family), is now routinely used in the clinic as a prognostic indicator and therapeutic target in breast cancer patients. Thus, continued advancement and development in this research topic may lead to the the development new chemopreventive, prognostic or therapeutic targets in malignant diseases.
ACKNOWLEDGEMENTS
I would like to express my gratitude to all those who gave me the opportunity to write this article. I wish to thank Dr. Subhas Chakrabarty for helpful discussion and critical reading of the manuscript. I would also like to give my thanks to Dr. Nie and Dr. Watabe for their support and valuable hints.
Abbreviations
- PGF
polypeptide growth factor
- EGFR
epidermal growth factor receptor
- FGF
fibroblast growth factor
- PDGF
platelet derived growth factor
- IGF
insulin-like growth factor
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
- ECM
extracellular matrix
- MMPs
metalloproteinases
- PTHrP
Parathyroid hormone related protein
- MSC
mesenchymal stem cell
- FAK
focal adhesion kinase
- MAPK
mitogen-activated protein kinase
- HGF
hepatocyte growth factor
- VSMC
vascular smooth muscle cell
- AR
androgen receptor
- EC
endothelial cell
REFERENCES
- 1.Wang H, Radjendirane V, Wary KK, Chakrabarty S. Transforming growth factor beta regulates cell-cell adhesion through extracellular matrix remodeling and activation of focal adhesion kinase in human colon carcinoma Moser cells. Oncogene. 2004;23:5558–5561. doi: 10.1038/sj.onc.1207701. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Chakrabarty S. Requirement of protein kinase Calpha, extracellular matrix remodeling, and cell-matrix interaction for transforming growth factorbeta-regulated expression of E-cadherin and catenins. J Cell Physiol. 2001;187:188–195. doi: 10.1002/jcp.1068. [DOI] [PubMed] [Google Scholar]
- 3.Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 2008 doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 4.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes FJ, Turner W, Belibasakis G, Martuscelli G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontol 2000. 2006;41:48–72. doi: 10.1111/j.1600-0757.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 7.Barr S, Thomson S, Buck E, Russo S, Petti F, Sujka-Kwok I, Eyzaguirre A, Rosenfeld-Franklin M, Gibson NW, Miglarese M, Epstein D, Iwata KK, Haley JD. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clin Exp Metastasis. 2008;25:685–693. doi: 10.1007/s10585-007-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol. 2007;27:3165–3175. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264:169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 10.Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, Vigneri R. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23–37. doi: 10.1080/13813450801969715. [DOI] [PubMed] [Google Scholar]
- 11.Peretz S, Kim C, Rockwell S, Baserga R, Glazer PM. IGF1 receptor expression protects against microenvironmental stress found in the solid tumor. Radiat Res. 2002;158:174–180. doi: 10.1667/0033-7587(2002)158[0174:irepam]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Normanno N, Gullick WJ. Epidermal growth factor receptor tyrosine kinase inhibitors and bone metastases: different mechanisms of action for a novel therapeutic application? Endocr Relat Cancer. 2006;13:3–6. doi: 10.1677/erc.1.01185. [DOI] [PubMed] [Google Scholar]
- 13.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. Embo J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 15.Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 16.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Franovic A, Gunaratnam L, Smith K, Robert I, Patten D, Lee S. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci U S A. 2007;104:13092–13097. doi: 10.1073/pnas.0702387104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007;26:333–339. doi: 10.1007/s10555-007-9063-1. [DOI] [PubMed] [Google Scholar]
- 19.Goldoni S, Iozzo RV. Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 20.Morinaga R, Okamoto I, Fujita Y, Arao T, Sekijima M, Nishio K, Ito H, Fukuoka M, Kadota JI, Nakagawa K. Association of epidermal growth factor receptor (EGFR) gene mutations with EGFR amplification in advanced non-small cell lung cancer. Cancer Sci. 2008 doi: 10.1111/j.1349-7006.2008.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon YK, Sung SW, Chung JH, Park WS, Seo JW, Kim CW, Chung DH. Clinicopathologic features and prognostic implications of epidermal growth factor receptor (EGFR) gene copy number and protein expression in non-small cell lung cancer. Lung Cancer. 2006;54:387–398. doi: 10.1016/j.lungcan.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9(Suppl 5):10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- 23.Swinson DE, O'Byrne KJ. Interactions between hypoxia and epidermal growth factor receptor in non-small-cell lung cancer. Clin Lung Cancer. 2006;7:250–256. doi: 10.3816/CLC.2006.n.002. [DOI] [PubMed] [Google Scholar]
- 24.Lieubeau-Teillet B, Rak J, Jothy S, Iliopoulos O, Kaelin W, Kerbel RS. von Hippel-Lindau gene-mediated growth suppression and induction of differentiation in renal cell carcinoma cells grown as multicellular tumor spheroids. Cancer Res. 1998;58:4957–4962. [PubMed] [Google Scholar]
- 25.Wang T, Niki T, Goto A, Ota S, Morikawa T, Nakamura Y, Ohara E, Ishikawa S, Aburatani H, Nakajima J, Fukayama M. Hypoxia increases the motility of lung adenocarcinoma cell line A549 via activation of the epidermal growth factor receptor pathway. Cancer Sci. 2007;98:506–511. doi: 10.1111/j.1349-7006.2007.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 27.Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol. 2000;76:589–605. doi: 10.1080/095530000138259. [DOI] [PubMed] [Google Scholar]
- 28.Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20:237–250. doi: 10.1023/a:1022939318102. [DOI] [PubMed] [Google Scholar]
- 29.Tang Y, Olufemi L, Wang MT, Nie D. Role of Rho GTPases in breast cancer. Front Biosci. 2008;13:759–776. doi: 10.2741/2718. [DOI] [PubMed] [Google Scholar]
- 30.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 31.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 32.LeBedis C, Chen K, Fallavollita L, Boutros T, Brodt P. Peripheral lymph node stromal cells can promote growth and tumorigenicity of breast carcinoma cells through the release of IGF-I and EGF. Int J Cancer. 2002;100:2–8. doi: 10.1002/ijc.10481. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki T, Nakamura T, Rebhun RB, Cheng H, Hale KS, Tsan RZ, Fidler IJ, Langley RR. Modification of the primary tumor microenvironment by transforming growth factor alpha-epidermal growth factor receptor signaling promotes metastasis in an orthotopic colon cancer model. Am J Pathol. 2008;173:205–216. doi: 10.2353/ajpath.2008.071147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pu J, McCaig CD, Cao L, Zhao Z, Segall JE, Zhao M. EGF receptor signalling is essential for electric-field-directed migration of breast cancer cells. J Cell Sci. 2007;120:3395–3403. doi: 10.1242/jcs.002774. [DOI] [PubMed] [Google Scholar]
- 35.Wei Q, Chen L, Sheng L, Nordgren H, Wester K, Carlsson J. EGFR, HER2 and HER3 expression in esophageal primary tumours and corresponding metastases. Int J Oncol. 2007;31:493–499. [PubMed] [Google Scholar]
- 36.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, Pinto A, Normanno N. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 37.Yi T, Lee HL, Cha JH, Ko SI, Kim HJ, Shin HI, Woo KM, Ryoo HM, Kim GS, Baek JH. Epidermal growth factor receptor regulates osteoclast differentiation and survival through cross-talking with RANK signaling. J Cell Physiol. 2008;217:409–422. doi: 10.1002/jcp.21511. [DOI] [PubMed] [Google Scholar]
- 38.Bruzzaniti A, Baron R. Molecular regulation of osteoclast activity. Rev Endocr Metab Disord. 2006;7:123–139. doi: 10.1007/s11154-006-9009-x. [DOI] [PubMed] [Google Scholar]
- 39.Zhu J, Jia X, Xiao G, Kang Y, Partridge NC, Qin L. EGF-like ligands stimulate osteoclastogenesis by regulating expression of osteoclast regulatory factors by osteoblasts: implications for osteolytic bone metastases. J Biol Chem. 2007;282:26656–26664. doi: 10.1074/jbc.M705064200. [DOI] [PubMed] [Google Scholar]
- 40.Normanno N, De Luca A, Aldinucci D, Maiello MR, Mancino M, D'Antonio A, De Filippi R, Pinto A. Gefitinib inhibits the ability of human bone marrow stromal cells to induce osteoclast differentiation: implications for the pathogenesis and treatment of bone metastasis. Endocr Relat Cancer. 2005;12:471–482. doi: 10.1677/erc.1.00956. [DOI] [PubMed] [Google Scholar]
- 41.Symons AL. Reduced growth hormone receptor immunoreactivity in osteoclasts adjacent to the erupting molar in the incisor-absent (osteopetrotic) rat. Eur J Oral Sci. 2003;111:503–509. doi: 10.1111/j.0909-8836.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 42.Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106:59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]
- 43.Angelucci A, Festuccia C, Gravina GL, Muzi P, Bonghi L, Vicentini C, Bologna M. Osteopontin enhances the cell proliferation induced by the epidermal growth factor in human prostate cancer cells. Prostate. 2004;59:157–166. doi: 10.1002/pros.20008. [DOI] [PubMed] [Google Scholar]
- 44.Hobisch A, Fiechtl M, Sandahl-Sorensen B, Godoy-Tundidor S, Artner-Dworzak E, Ramoner R, Bartsch G, Culig Z. Prostate cancer cells generated during intermittent androgen ablation acquire a growth advantage and exhibit changes in epidermal growth factor receptor expression. Prostate. 2004;59:401–408. doi: 10.1002/pros.10372. [DOI] [PubMed] [Google Scholar]
- 45.Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17:1–19. doi: 10.1016/s0945-053x(98)90121-4. [DOI] [PubMed] [Google Scholar]
- 46.Ruhland C, Schonherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. Febs J. 2007;274:4246–4255. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]
- 47.Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin. Down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem. 2000;275:35153–35161. doi: 10.1074/jbc.M006821200. [DOI] [PubMed] [Google Scholar]
- 48.Reed CC, Waterhouse A, Kirby S, Kay P, Owens RT, McQuillan DJ, Iozzo RV. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 49.Zhu JX, Goldoni S, Bix G, Owens RT, McQuillan DJ, Reed CC, Iozzo RV. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 50.Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT, McQuillan DJ, Iozzo RV. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 51.Kan M, Uematsu F, Wu X, Wang F. Directional specificity of prostate stromal to epithelial cell communication via FGF7/FGFR2 is set by cell- and FGFR2 isoform-specific heparan sulfate. In Vitro Cell Dev Biol Anim. 2001;37:575–577. doi: 10.1290/1071-2690(2001)037<0575:DSOPST>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 52.Nomura S, Yoshitomi H, Takano S, Shida T, Kobayashi S, Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Miyazaki M. FGF10/FGFR2 signal induces cell migration and invasion in pancreatic cancer. Br J Cancer. 2008;99:305–313. doi: 10.1038/sj.bjc.6604473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu X, Jin C, Wang F, Yu C, McKeehan WL. Stromal cell heterogeneity in fibroblast growth factor-mediated stromal-epithelial cell cross-talk in premalignant prostate tumors. Cancer Res. 2003;63:4936–4944. [PubMed] [Google Scholar]
- 54.Chesi M, Brents LA, Ely SA, Bais C, Robbiani DF, Mesri EA, Kuehl WM, Bergsagel PL. Activated fibroblast growth factor receptor 3 is an oncogene that contributes to tumor progression in multiple myeloma. Blood. 2001;97:729–736. doi: 10.1182/blood.v97.3.729. [DOI] [PubMed] [Google Scholar]
- 55.Tennant TR, Kim H, Sokoloff M, Rinker-Schaeffer CW. The Dunning model. Prostate. 2000;43:295–302. doi: 10.1002/1097-0045(20000601)43:4<295::aid-pros9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 56.Kwabi-Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer. 2004;11:709–724. doi: 10.1677/erc.1.00535. [DOI] [PubMed] [Google Scholar]
- 57.Matrisian LM, Cunha GR, Mohla S. Epithelial-stromal interactions and tumor progression: meeting summary and future directions. Cancer Res. 2001;61:3844–3846. [PubMed] [Google Scholar]
- 58.Yan G, Fukabori Y, McBride G, Nikolaropolous S, McKeehan WL. Exon switching and activation of stromal and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol Cell Biol. 1993;13:4513–4522. doi: 10.1128/mcb.13.8.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 60.Uematsu F, Jang JH, Kan M, Wang F, Luo Y, McKeehan WL. Evidence that the intracellular domain of FGF receptor 2IIIb affects contact of the ectodomain with two FGF7 ligands. Biochem Biophys Res Commun. 2001;283:791–797. doi: 10.1006/bbrc.2001.4850. [DOI] [PubMed] [Google Scholar]
- 61.Jin C, Wang F, Wu X, Yu C, Luo Y, McKeehan WL. Directionally specific paracrine communication mediated by epithelial FGF9 to stromal FGFR3 in two-compartment premalignant prostate tumors. Cancer Res. 2004;64:4555–4562. doi: 10.1158/0008-5472.CAN-03-3752. [DOI] [PubMed] [Google Scholar]
- 62.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 63.Smith K, Fox SB, Whitehouse R, Taylor M, Greenall M, Clarke J, Harris AL. Upregulation of basic fibroblast growth factor in breast carcinoma and its relationship to vascular density, oestrogen receptor, epidermal growth factor receptor and survival. Ann Oncol. 1999;10:707–713. doi: 10.1023/a:1008303614441. [DOI] [PubMed] [Google Scholar]
- 64.Weis SM, Lindquist JN, Barnes LA, Lutu-Fuga KM, Cui J, Wood MR, Cheresh DA. Cooperation between VEGF and beta3 integrin during cardiac vascular development. Blood. 2007;109:1962–1970. doi: 10.1182/blood-2005-10-038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicoli S, De Sena G, Presta M. Fibroblast Growth Factor 2-induced angiogenesis in zebrafish: the zebrafish yolk membrane (ZFYM) angiogenesis assay. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 67.Cao Y, Cao R, Hedlund EM. R Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J Mol Med. 2008;86:785–789. doi: 10.1007/s00109-008-0337-z. [DOI] [PubMed] [Google Scholar]
- 68.Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao X, Wetterskog D, Funa K, Brakenhielm E, Cao Y. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan W, Bentley B, Shao R. Distinct angiogenic mediators are required for basic fibroblast growth factor- and vascular endothelial growth factor-induced angiogenesis: the role of cytoplasmic tyrosine kinase c-Abl in tumor angiogenesis. Mol Biol Cell. 2008;19:2278–2288. doi: 10.1091/mbc.E07-10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Indraccolo S, Stievano L, Minuzzo S, Tosello V, Esposito G, Piovan E, Zamarchi R, Chieco-Bianchi L, Amadori A. Interruption of tumor dormancy by a transient angiogenic burst within the tumor microenvironment. Proc Natl Acad Sci U S A. 2006;103:4216–4221. doi: 10.1073/pnas.0506200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicoli S, Presta M. The zebrafish/tumor xenograft angiogenesis assay. Nat Protoc. 2007;2:2918–2923. doi: 10.1038/nprot.2007.412. [DOI] [PubMed] [Google Scholar]
- 72.Mitsiades CS, Hayden PJ, Anderson KC, Richardson PG. From the bench to the bedside: emerging new treatments in multiple myeloma. Best Pract Res Clin Haematol. 2007;20:797–816. doi: 10.1016/j.beha.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Betsholtz C, Karlsson L, Lindahl P. Developmental roles of platelet-derived growth factors. Bioessays. 2001;23:494–507. doi: 10.1002/bies.1069. [DOI] [PubMed] [Google Scholar]
- 74.Heldin CH, Eriksson U, Ostman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys. 2002;398:284–290. doi: 10.1006/abbi.2001.2707. [DOI] [PubMed] [Google Scholar]
- 75.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lederle W, Stark HJ, Skobe M, Fusenig NE, Mueller MM. Platelet-derived growth factor-BB controls epithelial tumor phenotype by differential growth factor regulation in stromal cells. Am J Pathol. 2006;169:1767–1783. doi: 10.2353/ajpath.2006.060120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, Keogh G, Merrett N, Pirola R, Wilson JS. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surg Res. 2008;149:319–328. doi: 10.1016/j.jss.2007.12.757. [DOI] [PubMed] [Google Scholar]
- 80.Sangai T, Ishii G, Kodama K, Miyamoto S, Aoyagi Y, Ito T, Magae J, Sasaki H, Nagashima T, Miyazaki M, Ochiai A. Effect of differences in cancer cells and tumor growth sites on recruiting bone marrow-derived endothelial cells and myofibroblasts in cancer-induced stroma. Int J Cancer. 2005;115:885–892. doi: 10.1002/ijc.20969. [DOI] [PubMed] [Google Scholar]
- 81.Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 82.McCarty MF, Somcio RJ, Stoeltzing O, Wey J, Fan F, Liu W, Bucana C, Ellis LM. Overexpression of PDGF-BB decreases colorectal and pancreatic cancer growth by increasing tumor pericyte content. J Clin Invest. 2007;117:2114–2122. doi: 10.1172/JCI31334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–846. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- 84.Chen Y, Miller C, Mosher R, Zhao X, Deeds J, Morrissey M, Bryant B, Yang D, Meyer R, Cronin F, Gostout BS, Smith-McCune K, Schlegel R. Identification of cervical cancer markers by cDNA and tissue microarrays. Cancer Res. 2003;63:1927–1935. [PubMed] [Google Scholar]
- 85.Gius D, Funk MC, Chuang EY, Feng S, Huettner PC, Nguyen L, Bradbury CM, Mishra M, Gao S, Buttin BM, Cohn DE, Powell MA, Horowitz NS, Whitcomb BP, Rader JS. Profiling microdissected epithelium and stroma to model genomic signatures for cervical carcinogenesis accommodating for covariates. Cancer Res. 2007;67:7113–7123. doi: 10.1158/0008-5472.CAN-07-0260. [DOI] [PubMed] [Google Scholar]
- 86.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee TJ, Sartor O, Luftig RB, Koochekpour S. Saposin C promotes survival and prevents apoptosis via PI3K/Akt-dependent pathway in prostate cancer cells. Mol Cancer. 2004;3:31. doi: 10.1186/1476-4598-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Werth C, Stuhlmann D, Cat B, Steinbrenner H, Alili L, Sies H, Brenneisen P. Stromal resistance of fibroblasts against oxidative damage: involvement of tumor cell-secreted platelet-derived growth factor (PDGF) and phosphoinositide 3-kinase (PI3K) activation. Carcinogenesis. 2008;29:404–410. doi: 10.1093/carcin/bgm296. [DOI] [PubMed] [Google Scholar]