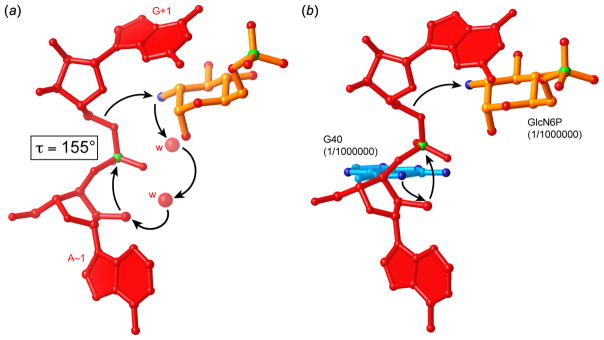
Figure 8.
Hypothetical catalytic mechanisms of the glmS ribozyme (Klein & Ferré-D’Amaré, 2006). (a) GlcN6P deprotonates the 2′-OH nucleophile through a proton relay employing two tightly bound water molecules, functioning as a general base catalyst. The resulting ammonium form of GlcN6P functions to stabilize the transition state electrostatically and also donates a proton to the 5′-oxo leaving group, functioning as a general acid catalyst. (b) The N1 imine of G40 functions as a general base catalyst. GlcN6P, in its ammonium form, functions as an electrostatic and general-acid catalyst. The ribozyme is inactive in the absence of GlcN6P or if a G40A mutation is made. The degree of impairment of the ribozyme indicated in parentheses is therefore an estimate based on the in vitro cleavage rate.