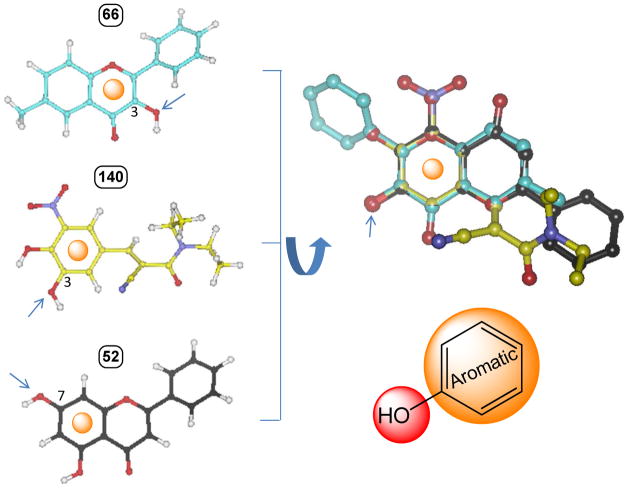
Figure 2. A flexible alignment of three most active substrates (66: 3-hydroxy-6-methylflavone, 140: entacapone; 52: chrysin) to identify their common structural features.
The flexible alignment was performed with a constraint that the glucuronidation site must be overlaid. The important commonalities of the most active substrates are found to be the glucuronidation site and its adjacent aromatic ring.Arrows indicate the site of glucuronidation.