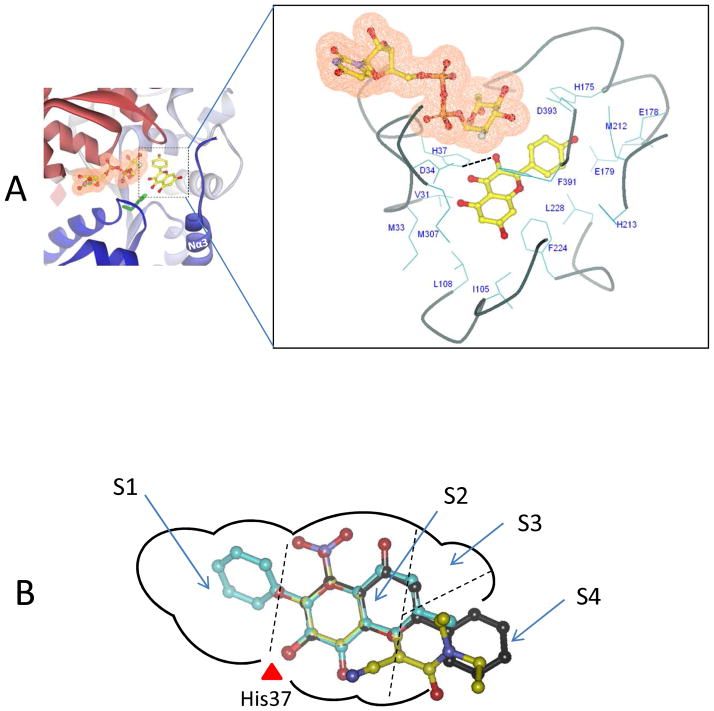
Figure 7. Three-dimensional model of the UGT1A9-kaempferol (88.a) complex.
Panel A , a side view of the three-dimensional model of UGT1A9-kaempferol complex. Kaempferol is indicated in a ball-and-stick model. The cofactor is indicated in a ball-and-stick model with a molecular surface. An expanded view of residues potentially involved in the interactions with kaempferol (3-O-glucuronidation) in the model is presented. Dashed black line indicates the potential hydrogen bond. Panel B, A two-dimensional schematic representation of UGT1A9 catalytic pocket. The three most active substrates (66: 3-hydroxy-6-methylflavone, 140: entacapone; 52: chrysin) are mapped into the pocket. The binding pocket is divided into four sub-pockets designated as S1, S2, S3 and S4.