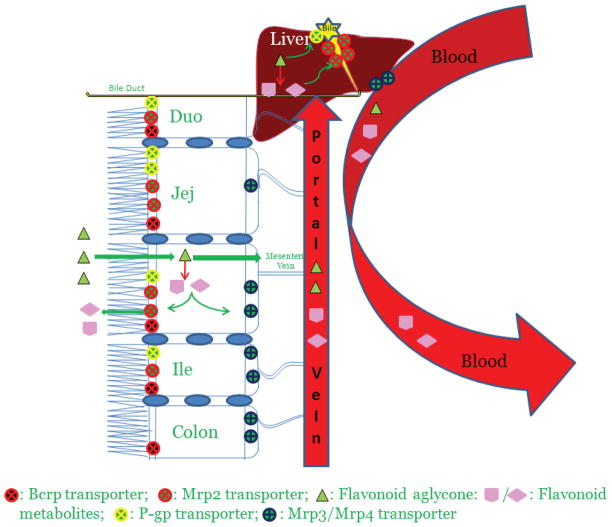
Figure 1.
Flow diagram depicting absorption and metabolism of flavonoids in rat intestine and liver. When taken orally, the aglycones shown here as triangles get absorbed in the enterocytes and undergo extensive phase II metabolism via various UGT isoforms. This first pass metabolism significantly reduces the flavonoid aglycone concentration reaching the systemic circulation thereby causing poor oral bioavailability. The flavonoid conjugates thus formed, being bulky and hydrophilic in nature require efflux transporters to get out of the cell on the luminal side by Mrp2 or on the serosal side by Mrp3 and 4. Flavonoid aglycone may escape the intestinal metabolism and make its way into the liver through the portal vein. Similar to intestine, flavonoid aglycone is rapidly and extensively glucuronidated by various UGT isoforms in liver and the conjugates are excreted either into the bile (major pathway) via Mrp2 transporters or into the systemic circulation (minor pathway) by membrane-bound Mrp3 and Mrp4 transporters. The bile along with flavonoid conjugates is then emptied in the upper part of the duodenum. The flavonoid conjugates thus excreted from liver and from the upper part of the intestine (duodenum and jejunum) make their way into the colon. The bacterial microflora present in the lower part of the small intestine and colon hydrolyzes these conjugates back into flavonoid aglycone which gets absorbed through the colon back into the systemic circulation. These recycling mechanisms called enteric and enterohepatic recycling thereby improve apparent plasma half-life of flavonoids in rats.