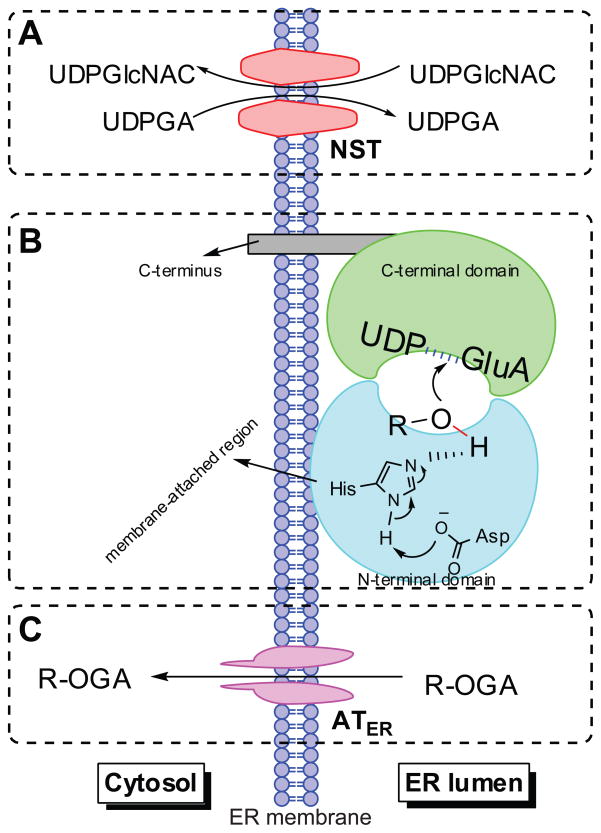
Figure 3.
Schematic representation of human UGT topology. UGTs consist of two domains (panel B) and are predicted to function as dimers or oligomers. The amino-terminal domain binds the aglycone and the carboxy-terminal domain binds the UDPGA cofactor; the catalytic site is placed between the two domains. Most of the enzyme mass is located on the luminal side of the endoplasmic reticulum and the carboxy-terminal tail is on the cytosolic side of the membrane. Transport of the cofactor UDPGA into the ER lumen requires the nucleotide sugar transporters (NSTs) (panel A), which act as antiporters counter-transporting the UDP-N-acetyl glucosamine (UDPGlcNAC) out of ER lumen. Whereas, the translocation of the formed glucuronide(s) to the cytosol is suggested to be mediated by ER-localized organic anion transporters (ATER), which do not need ATP, but transport organic anions through ER membranes by facilitated diffusion (panel C). R-OH: phenolics. R-OGA: glucuronide.