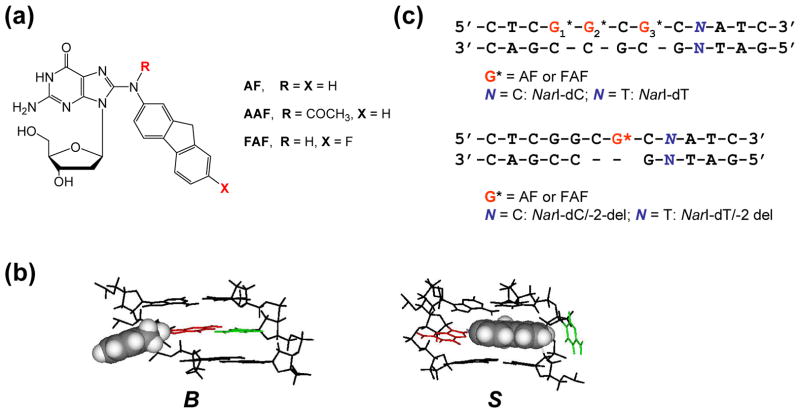
Figure 1.
(a) Chemical structures of AF-adduct [N-(2′-deoxyguanosin-8-yl)-2-aminofluorene], AAF-adduct [N-(2′-deoxyguanosin-8-yl)-2-acetylaminofluorene], and FAF-adduct [N-(2′-deoxyguanosin-8-yl)-7-fluoro-2-aminofluorene]. (b) The major groove views of the central trimer segments of the B- and S-conformers of AF-modified duplex. The modified dG and the complementary dC are shown in red and green, respectively, and the AF-moiety is highlighted with grey CPK. In the B conformer, anti-[AF]dG maintains Watson-Crick hydrogen bonds, thereby placing the AF ring in the major groove. The AF moiety of the S-conformer stacks into the helix with the modified dG in the syn conformation. (c) Sequence contexts of the fully-paired and -2 deletion NarI duplexes.