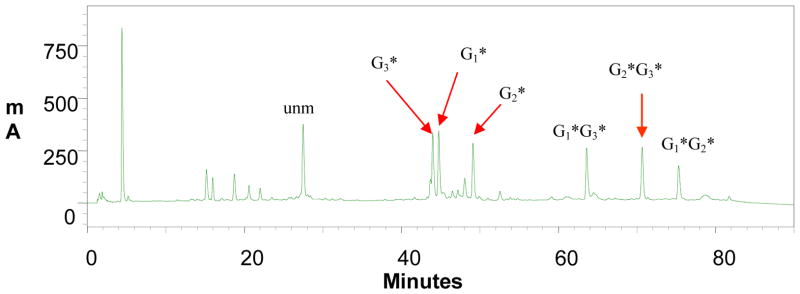
Figure 2.
HPLC chromatogram of a mixture from reaction between NarI-dC 12-mer sequence and an activated FAF (N-trifluoroacetyl-N-acetoxy-7-fluoro-2-aminofluorene). The mono- (G3*, G1*, G1*) and di- (G1*G3*, G2*, G3*, G1*G2*) FAF adducts eluted in the 40–50 and 60–80 min ranges were purified by reverse HPLC and characterized by LC-TOF-MS. The HPLC system consisted of Waters Xterra 5 m colum with flow rate of 2 mL/min. A gradient condition was 3–15% acetonitrile in triethylammonium acetate buffer (pH 7.0, 100 mM) for 60 min, followed by 15%–20% for 20 minutes and back to 3% acetonitrile in 10 min, flow rate 2 ml/min.