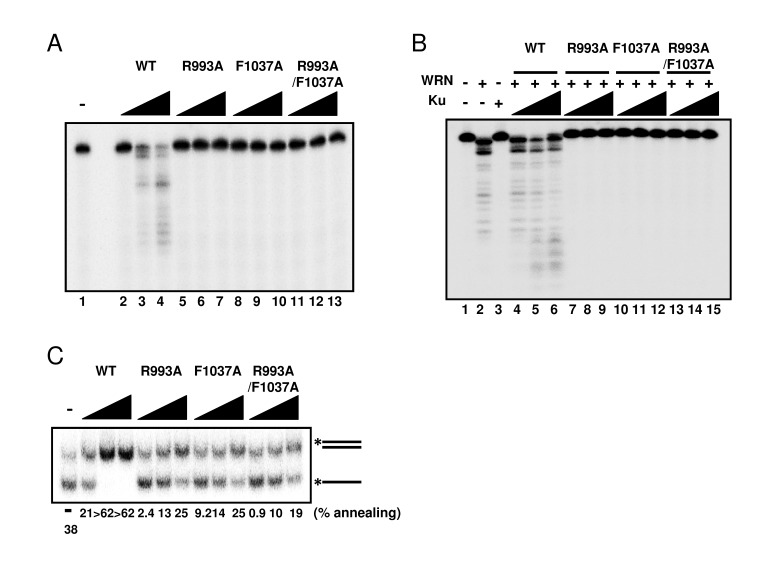
Figure 5. Mutations in RQC domain of WRN result in significant decrease of exonuclease activity and strand annealing activity.
(A) Exonuclease activity of WRN mutants was severely reduced. 5, 10, 20 nM WRN wild type (lane 2 to 4) or WRN variants (lane 5 to 7, R993A; lane 8 to 10, F1037A; lane 11 to 13, R993A/F1037A) were incubated with 0.5 nM 5' overhang duplex substrate. Products were separated on 14 % denaturing polyacrylamide gel. (B) Ku70/80 heterodimeric protein is not able to stimulate exonuclease activity of WRN mutants. 10 nM WRN wild type (lane 4 to 6) or WRN mutants (lane 7 to 9, R993A; lane 10 to 12, F1037A; lane 13 to 15, R993A/F1037A) were incubated with 5, 10, 20 nM Ku heterodimer and substrate. (C) WRN variants exhibit significantly lower strand annealing activity than the WRN wild type protein. 1, 2, 5 nM WRN wild type or WRN RQC variants were incubated with 0.5 nM DNA substrate at 37 °C for 15 min.