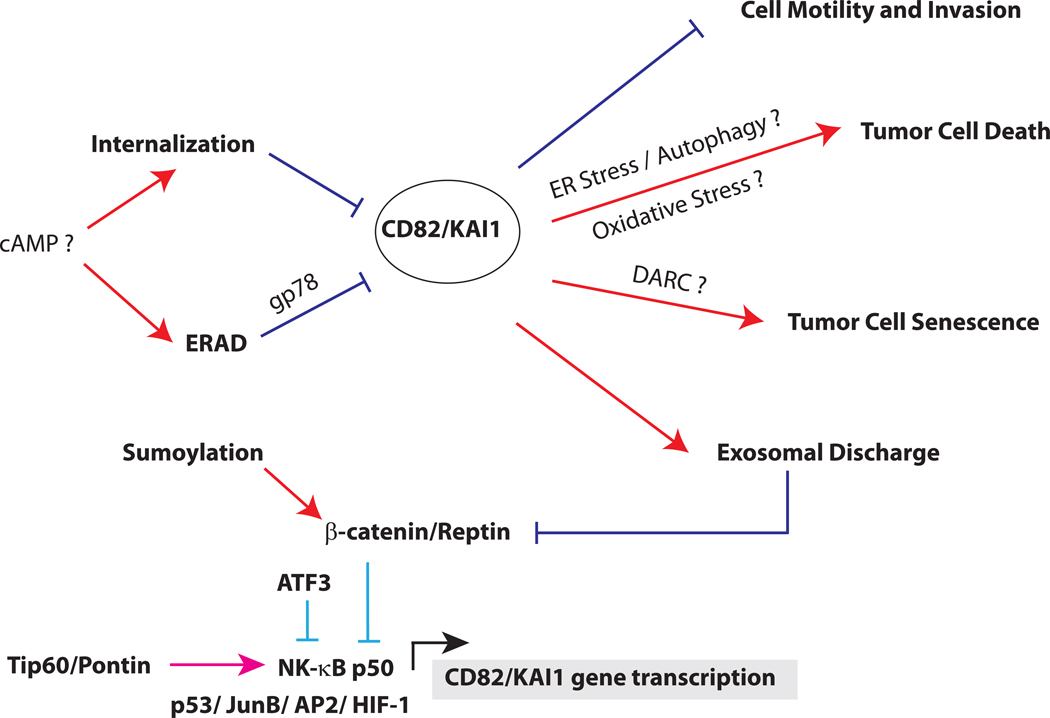
Figure 2. Schematic of CD82 regulation and functions.
CD82 can be transcriptionally activated by p53, JunB, AP2, NF-κB p50 and HIF-1 (below). CD82 expression by p50 is regulated by the alternative recruitment of Tip60/Pontin or β-catenin/Reptin complex. In normal cells, CD82 transcription is repressed by a NCOR/TAB2/HDAC3 complex (not shown), which is displaced by a Tip60/Pontin co-activator complex for p50 in the presence of cytokines. In cancer cells, Tip60 is downregulated and replaced with a β-catenin/Reptin complex, which represses CD82 transcription. Sumoylation of Reptin is required for its repressor function, which can be inactivated by de-sumoylation. ATF3 can also repress p50 induction of CD82. After translation (above), CD82 protein is regulated by quality control mechanisms in the endoplasmic reticulum. This process, known as ER-associated degradation (ERAD), depends on the ubiquitin ligase gp78. In some cancer cells, cAMP promotes CD82 degradation either by enhancing its internalization or accelerating its degradation from the ER. CD82 inhibits metastasis by multiple mechanisms, including inhibiting cell motility and invasion, promoting apoptosis, inducing tumor cell senescence and enhancing exosomal discharge of β-catenin.