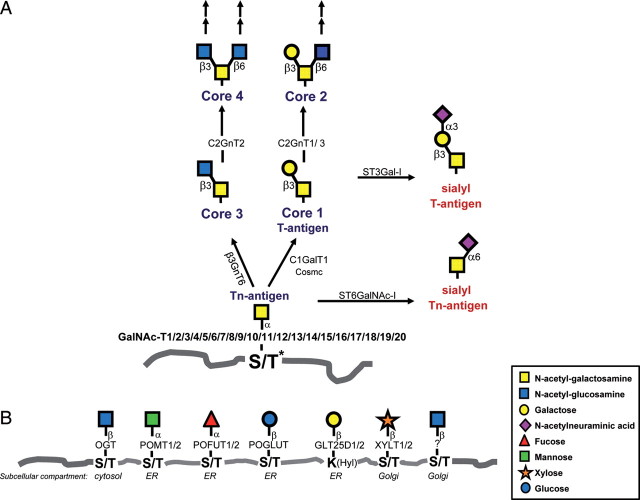
Fig. 1.
Mammalian protein O-glycosylation pathways. (A) The common mucin-type O-glycosylation core 1–4 biosynthetic pathways. Mucin-type O-glycosylation is initiated by up to 20 GalNAc-Ts forming the Tn structure, which may be elongated by the core 1 synthase, C1Gal-T1, or the core 3 synthase, β3GnT6, and further branched by the core 2 synthases, C2GnT1-3. C1Gal-T1 function is dependent on the presence of the chaperone COSMC. The different core structures can be further elongated and branched by N-acetyllactosamine chains and/or terminated by blood group ABH-related structures, fucose and sialic acids. Sialylation may terminate chain elongation and branching as indicated by the action of ST3Gal-I on core 1, which produces the ST structure. Premature sialylation of the first GalNAc by ST6GalNAc-I leads to the cancer-associated structure STn. (Asterisk) Recent studies demonstrate that GalNAc may also be bound to Tyr (Halim et al. 2011; Steentoft et al. 2011). (B) Other known types of protein O-glycosylation in mammals and the initiating enzymes. These types include O-GlcNAc found on nuclear and cytoplasmic proteins, O-mannose found on α-dystroglycan, O-fucose and O-glucose found on EGF domains in membrane proteins, O-Gal linked to 5-Hyls found on collagens, O-xylose found on proteoglycans and recently identified O-GlcNAc found on extracellular proteins (Matsuura et al. 2008; Sakaidani et al. 2010). Glycosyltransferases involved in the formation of the structures depicted are indicated by their official name, and the subcellular compartments where these modifications are initiated are indicated.