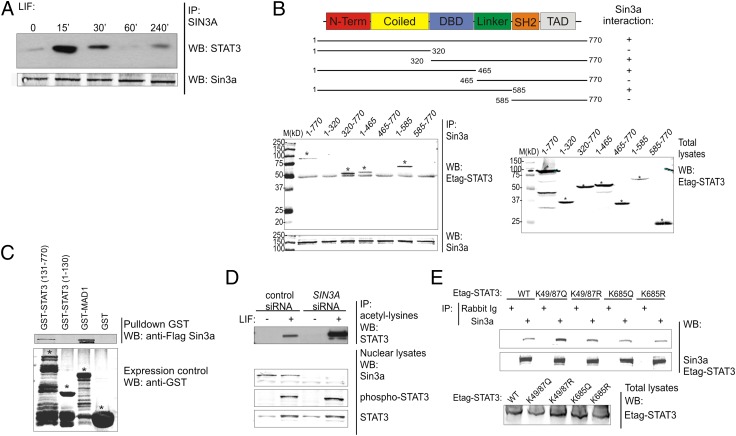
Fig. 2.
Sin3a interacts with STAT3 and modifies its acetylation pattern. (A) Hek293T cells were cultured 4 h without FCS and then stimulated with LIF for the indicated time points. Nuclear lysates were prepared, endogenous Sin3a was immunoprecipitated, and coprecipitated endogenous STAT3 was revealed with an anti-STAT3 antibody. Precipitated Sin3a was revealed using an anti-Sin3a antibody. (B) Schematic representation of Etag-STAT3 truncated mutants. DBD, DNA binding domain; SH2, Src Homology 2; TAD, transactivation domain. Hek293T cells were transfected with plasmids coding for GFP-Sin3a and Etag-STAT3 truncated mutants. Sin3a was immunoprecipitated and coprecipitated Etag-STAT3 mutants were revealed with anti-Etag antibody. Total lysates were blotted as loading control. Stars indicate the specific bands. (C) Escherichia coli BL21DE3 cells were transformed with plasmids coding for GST-STAT3 N-terminal (1-130), C-terminal (131-770), GST-MAD1 (positive control), or GST alone (negative control). Cell lysates were incubated with the in vitro transcribed and translated Flag-Sin3a protein. Complexes were precipitated using glutathione-agarose beads and revealed with anti-flag antibody. GST-transformed bacterial lysates were blotted with anti-GST antibody as control of protein production and solubility. Stars indicate the specific bands. (D) Hek293T cells were transfected with control (Renilla luciferase) or SIN3A siRNA. After 72 h, cells were cultured 4 h without FCS and then stimulated with LIF for 30 min. Nuclear extracts were prepared and acetylated lysines were immunoprecipitated. Coprecipitated STAT3 was revealed with anti-STAT3 antibody. Endogenous Sin3a and STAT3 levels in the nuclear lysates were detected using specific antibodies. (E) Hek293T cells were transfected with plasmids coding for GFP-Sin3a and Etag-STAT3 WT, acetyl-mimicking (K49/87Q and K685Q), or acetyl-deficient (K49/87R and K685R) mutants. Sin3a was immunoprecipitated and coprecipitated Etag-STAT3 mutants were revealed with an anti-Etag antibody. Total lysates were blotted as loading control. Results are representative of three independent experiments.