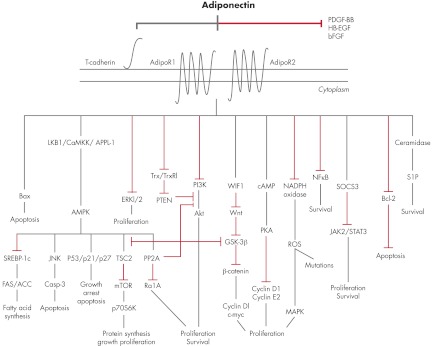
Figure 1.
Signaling pathways connecting adiponectin to carcinogenesis. Adiponectin may act on cancer tissues either by sequestrating growth factors at the prereceptor level or by binding to AdipoR1, AdipoR2, and T-cadherin. T-Cadherin has not been associated with downstream effector molecules and may serve as a coreceptor for adiponectin. Binding to AdipoR1 and AdipoR2, initiates a cascade of signaling molecules comprising, among others, activation of AMPK by the cofactors LKB1, APPL-1, and/or CaMKK; induction of Bax and cAMP/PKA; as well as inhibition of ERK1/2, PI3K/Akt, Wnt/β-catenin, nicotinamide adenine dinucleotide phosphate-oxidase/ROS/MAPK, NF-κB, Bcl-2, and JAK2/STAT3 signaling. Activated AMPK subsequently stimulates JNK, PP2A, and the cell cycle regulators p53/p21/p27, while negatively influencing fatty acid synthase (FAS)/ACC and the mTOR/S6K axis. Collectively, these effects result in reduced fatty acid and protein synthesis; decreased cellular growth, proliferation, and DNA-mutagenesis; and increased cell cycle arrest and apoptosis, thus negatively influencing carcinogenesis. Cross talk between the mentioned pathways adds further complexity to the adiponectin-induced signaling network. Finally, recent advances show that adiponectin can enhance ceramidase activity independently from AMPK via AdipoR1/R2, contributing to increased amounts of prosurvival S1P. The black and red lines indicate stimulatory and inhibitory effects, respectively. Trx, Thioredoxin.