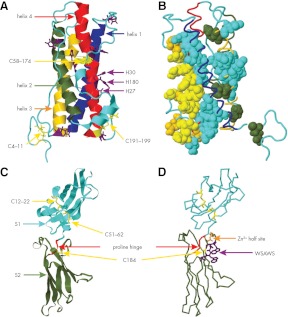
Figure 1.
Structures of hPRL and the EC domain of the hPRL receptor. A, hPRL, PDB no. 1RW5 (ribbon). Helix 1, residues 14–43 (blue); helix 2, residues 78–103 (green); helix 3, residues 111–137 (gold); and helix 4, residues 161–193 (red). Disulfides (yellow stick), residues 4–11, 58–174, and 191–195. Histidines (purple stick). B, hPRL (cyan backbone); residues displaying significant ΔΔG when mutated to alanine (solid). Solid gold, site 1 residues; solid yellow, site 1 residues noncovalently linked to the hPRL receptor. Site 1, Loop 1 (I55, N56, P66, E67, and Q73) and helix 4 (R177, H180, K181, D183, N184, K187, R192, N197, and N198). Solid green, site 2; helix 1 (Q12), and helix 3 (Q122, L126, and L132). Solid cyan, Coupling motif; helix 1 (F19, V23, S33, S34, and F37), loop 1 (S57 and L63), helix2/helix 3 junction (E110), helix 3 (E118, I119, E120, E121, T123, K124, and L127), helix 4 (Y169, N170, D178, S179, L189, and I193), and C terminus (I194 and N196). C, EC domain of the hPRL receptor (PDB no. 3NO6, ribbon residues 2–204). Cyan, S1 subdomain; green, S2 subdomain; yellow stick, cysteines (184) and disulfides (12–22, 51–62); red, proline hinge (PDPP, residues 103–106). D, EC domain of the hPRL receptor; yellow stick, cysteine 184; gold stick, Zn2+ half-site (gold stick residues D187 and H188); and blue stick, WSAWS motif (residues W191, S192, A193, W194, and S195).