Abstract
Context:
Both training and normal body mass index are associated with high insulin sensitivity, but the mechanism may be different.
Objective:
The aim of the study was to examine whether lean trained humans may be protected from acute free fatty acid (FFA)-induced insulin resistance compared with lean sedentary humans.
Design and Setting:
We conducted an interventional trial using either a 6-h lipid (20% Intralipid at 90 ml/h) or glycerol (2.25 g/100 ml at 90 ml/h) infusion along with a concurrent hyperinsulinemic-euglycemic clamp and serial muscle biopsies (0, 120, 360 min) at a clinical research unit at the University of Minnesota.
Patients or Participants:
The study included lean endurance-trained (n = 14) and sedentary (n = 14) individuals matched for age, gender, and body mass index.
Main Outcome Measures:
We measured the decline in glucose infusion rate (GIR) during the hyperinsulinemic-euglycemic clamp.
Results:
The trained group had higher baseline mitochondrial DNA copy number, mRNA of cytochrome C oxidase subunit 3, and insulin sensitivity (as measured by GIR) compared with the sedentary group. When FFA was acutely elevated to the upper physiological range (0.6–0.7 mEq/liter) by lipid infusion, the GIR in both activity groups declined similarly compared with their respective glycerol controls, although insulin signaling, as measured by Ser 473 pAKT/AKT, remained comparable. Specific to the trained group, the stimulatory effect of hyperinsulinemia on mitochondrial mRNA levels during the glycerol infusion was absent during the lipid infusion.
Conclusions:
Elevated FFA had similar effects in reducing insulin sensitivity in trained and sedentary humans. In trained participants, this decline was associated with alterations in the skeletal muscle mitochondrial mRNA response to hyperinsulinemia.
Insulin resistance plays a critical role in the development of type 2 diabetes, with skeletal muscle the largest site of insulin resistance in the human body (1). Reduced body fat and exercise are both associated with reduced insulin resistance (2, 3) and lower risk for type 2 diabetes (4, 5), yet whether these similar outcomes are mediated by similar mechanisms remains unclear.
Several lines of evidence suggest that training may modify skeletal muscle mitochondrial function to improve resting skeletal muscle insulin resistance. It is well established that in sedentary participants, insulin resistance correlates with intramyocellular lipid (IMCL) levels (6) and accumulation of lipid metabolites (7, 8). In contrast, lean endurance-trained participants have IMCL levels comparable to those of patients with type 2 diabetes but remain insulin sensitive (9). Given that mitochondria is a major site of lipid metabolism and training enhances muscle mitochondrial function (10) and biogenesis (11), training may protect against the adverse effects of lipotoxicity. When sedentary obese humans participated in a 16-wk aerobic training program, the improvement in insulin sensitivity was associated with increased skeletal muscle oxidative capacity, increased IMCL, and reduced lipid metabolites (3).
Although training may protect skeletal muscle from the metabolic effects of lipid accumulation, whether trained individuals are protected from the metabolic effect of acute free fatty acid (FFA) elevation remains unknown, particularly because previous studies in trained individuals lacked a glycerol control (12, 13). Lipid infusion is an established model of FFA-induced insulin resistance (14–16). The early studies using lipid infusion to produce insulin resistance also coadministered heparin, resulting in FFA elevation to supraphysiological levels (14, 15). Subsequently, it has been demonstrated that the effect of FFA elevation on peripheral insulin resistance is dose-related and is present even with FFA elevation within the physiological range (16).
The aim of this study was to examine differences in resting insulin sensitivity between healthy lean trained and lean sedentary individuals and whether training may modify the effect of acute FFA elevation within the physiological range on skeletal muscle insulin sensitivity. We hypothesized that lean trained participants may be protected from acute FFA-induced insulin resistance compared with lean sedentary humans.
Subjects and Methods
Healthy lean trained (n = 14) and sedentary (n = 14) individuals were recruited and gave written informed consent. Individuals were screened using a detailed medical history, hematological, and biochemical profile. Participants were matched for gender, age (±5 yr), and body mass index (BMI) (±1.5 kg/m2). Training was self-reported using the short-form International Physical Activity Questionnaire, a validated physical activity questionnaire (17). Individuals who participated in 30 min or less of active exercise weekly were classified as sedentary. Individuals who participated in a regular running program (minimum >45 min/d at least 5 d/wk) preferably with recent marathon experience, were classified as trained. Exclusion criteria included: use of any medications that may affect lipid levels; high dietary fat intake (>45%) as measured by screening questionnaire (18); any impediment to exercise testing as measured by screening questionnaire (19); pregnancy; recent weight change (>5 pounds within the last 3 months); high fasting triglyceride concentration (≥3.36 mmol/liter); high fasting glucose level (≥5.6 mmol/liter); allergy to egg, soy, or a lipid emulsion; use of anticoagulation or antiplatelet medication; or significant hematological, hepatic, renal pulmonary, or cardiac abnormalities noted at screening.
Protocol
The protocol was approved by the University of Minnesota Institutional Review Board. Visits were conducted at the Masonic Clinical Research Unit (MCRU). Before each visit, participants abstained from active exercise (minimum 48 h) and food intake (8 h). Female volunteers were studied during the follicular phase of their menstrual cycle.
Participants were screened at visit 1, which included a fasting blood draw, height, weight, electrocardiogram, and treadmill maximal oxygen consumption (VO2 max) (Medgraphics CPX-D metabolic cart, Medical Graphics Corporation, St. Paul, MN).
Visit 2 took place at least 1 wk and no more than 1 month after visit 1. Body composition was measured by dual-energy x-ray absorptiometry (Lunar Prodigy; GE Healthcare, Madison, WI) followed by a 3-h hyperinsulinemic-euglycemic clamp to measure insulin sensitivity. An insulin infusion was started [1.5 mU/kg fat free mass (FFM)/min] along with a potassium infusion (KPO4 at 50 ml/hr) and glucose infusion (dextrose 20%, titrated to maintain the blood glucose at 4.7–5.3 mmol/liter) based on bedside glucose measured every 10 min (Analox model GM9D; Analox Instruments, Lunenburg, MA).
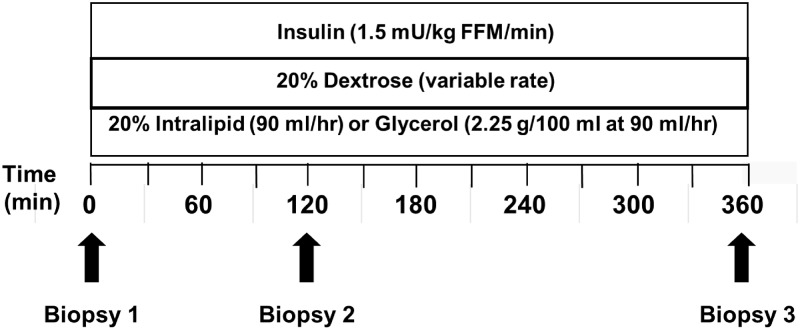
Visit 3 took place within 1 month of the second visit. Participants arrived at the MCRU in the evening, consumed a standard dinner (27% protein, 32% fat, 41% carbohydrate), remained on bed rest with bathroom privileges, and consumed no other food until study completion. At 0800 h the next day (Fig. 1), participants underwent a muscle biopsy under local anesthesia on the right vastus lateralis (20). Approximately 200 mg of muscle was obtained, trimmed of excess fat at the bedside, frozen in liquid nitrogen-cooled isopentane, and then stored at −80 C. After the first biopsy was obtained, a 6-h infusion of lipid (20% Intralipid at 90 ml/h) or glycerol (2.25 g/100 ml at 90 ml/h) was started. To limit the effect of the lipid infusion to FFA elevation, the control infusion was a glycerol infusion matched to the glycerol content of the lipid infusion. Participants were assigned to either a lipid or glycerol infusion to maintain matching between activity groups. In addition, a hyperinsulinemic-euglycemic clamp was started (insulin, 1.5 mU/kg FFM/min; KPO4 at 50 ml/h; dextrose 20% titrated to keep glucose at 4.7–5.3 mmol/liter) and continued for 6 h. Blood was taken every 30 min for measurement of insulin and FFA levels. After initiating the lipid/glycerol infusion, biopsy 2 (120 min) was obtained from the right vastus lateralis, 6 inches proximal to the first biopsy site, and biopsy 3 (360 min) muscle biopsy was obtained from the left vastus lateralis. After biopsy 3 was completed, all infusions were discontinued. The participant was fed and dismissed from the MCRU.
Fig. 1.
Outline of visit 3. Bedside glucose was measured every 10 min for adjustment of the GIR. Blood was collected every 30 min for measurement of glucose, insulin, and FFA. Three muscle biopsies were obtained from the vastus lateralis (biopsy 1, time 0 min; biopsy 2, time 120 min; biopsy 3, time 360 min) over the course of the lipid/glycerol infusion.
Measurements
Plasma glucose, insulin, and FFA
During the hyperinsulinemic-euglycemic clamps, glucose was measured immediately at the bedside every 10 min (Analox model GM9D). Plasma insulin was determined by radioimmunoassay (21). Nonesterified free fatty acids were measured with an enzymatic colorimetric assay (NEFA C; Wako Chemicals, Richmond, VA).
Insulin sensitivity
Insulin sensitivity was calculated from the glucose infusion rate (GIR) required to maintain plasma glucose (4.7–5.3 mmol/liter) during the last 30 min of the hyperinsulinemic-euglycemic clamp (21) and normalized to FFM (22). For visit 2, GIR was calculated from time 150–180 min. For visit 3, GIR was calculated from time 330–360 min. The area under the curve (AUC) for the GIR was calculated from time 0 to time 360 min.
AMP kinase (AMPK) phosphorylation
AMPK phosphorylation was measured using antibodies against AMPK (catalog no. 2532; Cell Signaling Technology, Danvers, MA) and phosphorylated AMPKα (Thr172) (pAMPK) (catalog no. 2531) that were diluted at 1:1000 and developed using antirabbit-horseradish peroxidase secondary (catalog no. 170-6515; Bio-Rad Laboratories Inc., Hercules, CA).
Insulin signaling
Insulin signaling was measured using antibodies against insulin receptor substrate-1 (IRS1) (catalog no. 2382; Cell Signaling Technology), phosphorylated IRS1 (pIRS1) (Ser 636/639, no. 2388), AKT (no. 9272), and pAKT (Ser 473, no. 9271), and developed using antirabbit-horseradish peroxidase secondary (catalog no. 170-6515; Bio-Rad Laboratories Inc.).
Muscle mitochondrial DNA (mtDNA) and mRNA
DNA and RNA extractions were performed from each frozen skeletal muscle biopsy (11). For quantification of mtDNA, established primer and probe sequences for NADH dehydrogenase subunits 1 and 4 (ND1 and ND4) were used. The measured mtDNA was normalized to 28S ribosomal RNA (23). For quantification of mRNA related to mitochondrial genes, established primers, and probe sequences against cytochrome C oxidase subunit 3 (COX3), ND4, mitochondrial transcription factor A (TFAM), peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1a), and nuclear respiratory factor 1 (NRF1) were used and normalized to 28S ribosomal RNA (11).
Statistical analysis
Data are reported as means ± se in Table 1 and Fig. 2 and as least square means ± se in Figs. 3 and 4. Due to matching within (lipid vs. glycerol) and between activity (trained vs. sedentary) groups, significant differences were determined using paired, two-tailed t tests. Multiple group comparisons were performed: 1) trained lipid vs. trained glycerol; 2) sedentary lipid vs. sedentary glycerol; 3) trained lipid vs. sedentary lipid; and 4) trained glycerol vs. sedentary glycerol. Statistical significance was set at P < 0.05. When examining the trajectories of measured values over time, a mixed model analysis with a random participant effect were used to evaluate the infusion group by time interaction. All analyses were conducted with SAS (version 9.2; SAS Institute, Inc., Cary, NC).
Table 1.
Baseline characteristics
| Trained | Sedentary | P value | |
|---|---|---|---|
| n | 14 (8 males, 6 females) | 14 (8 males, 6 females) | |
| Age (yr) | 23.0 (0.8) | 21.6 (0.2) | 0.21 |
| BMI (kg/m2) | 22.6 (0.6) | 21.5 (0.6) | 0.22 |
| FFM (kg) | 54.0 (3.4) | 44.0 (1.9) | 0.01 |
| Body fat (%) | 18.4 (1.8) | 25.6 (2.48) | 0.06 |
| Fasting glucose (mmol/liter) | 4.4 (0.1) | 4.6 (0.1) | 0.45 |
| FFA (mEq/liter) | 0.28 (0.06) | 0.41 (0.06) | 0.14 |
| Triglycerides (mmol/liter) | 1.01 (0.08) | 0.78 (0.07) | 0.02 |
| Insulin (pmol/liter) | 14.4 (4.2) | 30 (5.4) | 0.05 |
| VO2 max (ml/kg/min) | 49.2 (1.8) | 39.0 (1.4) | <0.01 |
| GIR (μmol glucose infused/kg FFM/min) | 71.6 (5.3) | 49.4 (3.8) | <0.01 |
| Ser 636/639-pIRS1/IRS1 | 0.80 (0.09) | 0.93 (0.10) | 0.37 |
| Ser 473-pAKT/AKT | 0.98 (0.26) | 1.01 (0.25) | 0.92 |
| pAMPK/AMPK | 0.97 (0.63) | 1.29 (0.92) | 0.35 |
| mtDNA copy no. (ND1/28S) | 1.58 (0.29) | 0.81 (0.30) | 0.08 |
| mtDNA copy no. (ND4/28S) | 1.15 (0.13) | 0.70 (0.14) | 0.03 |
| COX3 mRNA/28S | 0.70 (0.09) | 0.36 (0.09) | 0.02 |
| ND4 mRNA/28S | 0.70 (0.12) | 0.47 (0.12) | 0.2 |
| TFAM mRNA/28S | 0.51 (0.04) | 0.53 (0.05) | 0.79 |
| PGC-1a mRNA/28S | 0.65 (0.06) | 0.61 (0.06) | 0.64 |
| NRF1 mRNA/28S | 0.76 (0.07) | 0.94 (0.07) | 0.11 |
Data are expressed as means ± se. P value indicates a two-sample t test comparison between the trained and sedentary groups at their baseline assessment. Boldface P values indicate statistically significant difference between the trained and sedentary groups.
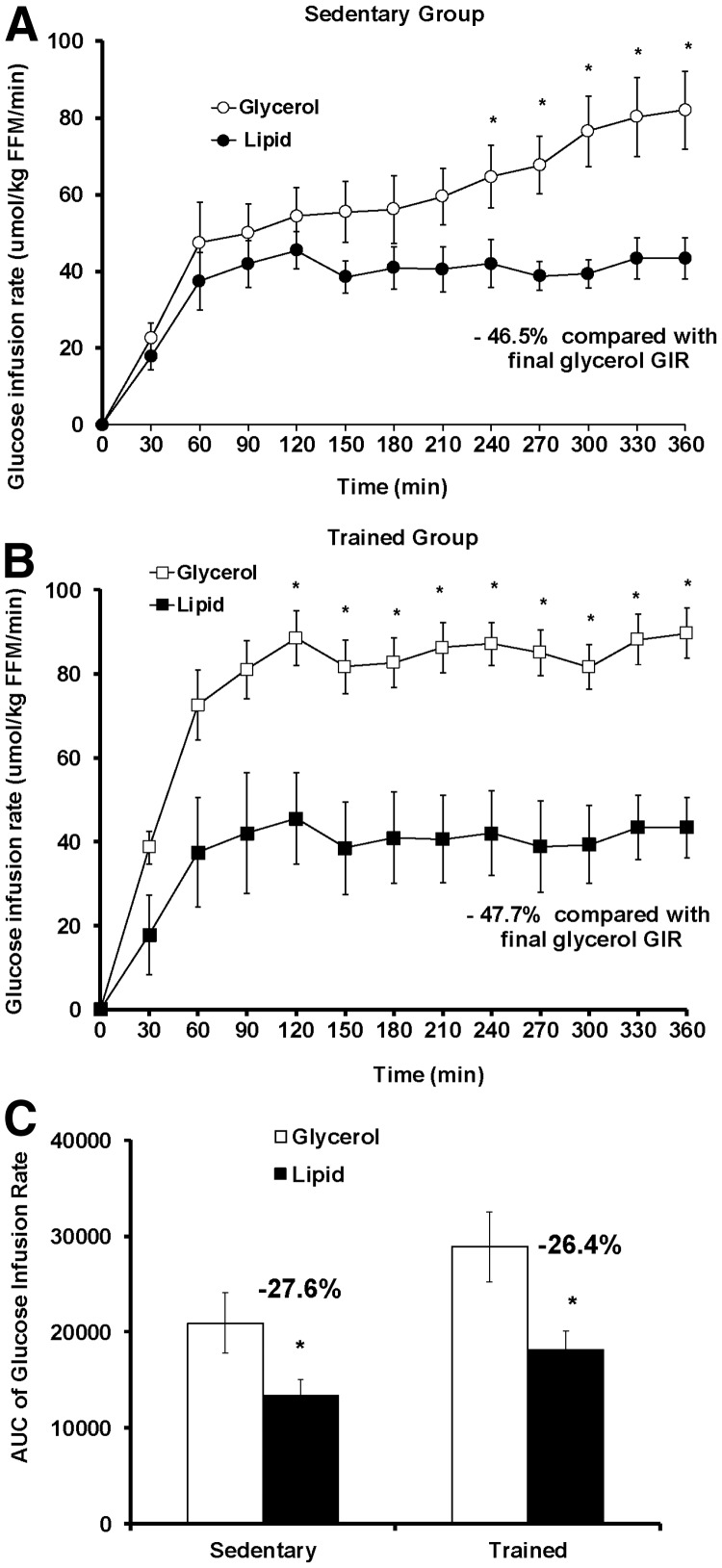
Fig. 2.
GIR during a 6-h hyperinsulinemic-euglycemic clamp with concurrent lipid or glycerol infusion. The GIR required for maintenance of euglycemia during the 6-h clamp was higher in the glycerol group compared with the lipid group in both the sedentary (A) and trained (B) subjects. C, Lipid infusion produced a greater decline in the GIR AUC during the entire clamp (0–360 min) in the trained group than the sedentary group. Values shown are mean ± se. *, Significant (P < 0.05) difference for the lipid vs. glycerol comparison within an activity group. White circle, Sedentary glycerol group (n = 7); black circle, sedentary lipid group (n = 7); white square, trained glycerol group (n = 7); black square, trained lipid group (n = 7).
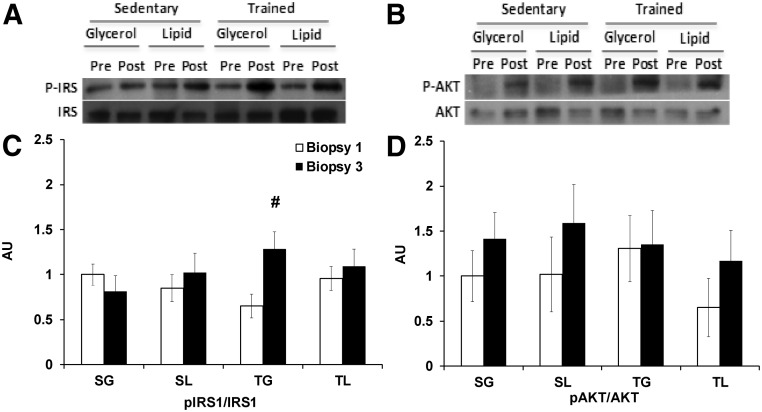
Fig. 3.
Alterations in insulin signaling during the 6-h hyperinsulinemic-euglycemic clamp. Insulin signaling was measured by Ser 636/639-pIRS1/IRS1 (A and C) and Ser 473-pAKT/AKT (B and D) at biopsy 1 (0 min) and biopsy 3 (360 min). A and B, Representative Western blots. C and D, Sedentary glycerol (SG; n = 7), sedentary lipid (SL; n = 7), trained glycerol (TG; n = 7), and trained lipid (TL; n = 7) groups are shown. #, P < 0.05 for the paired comparison with biopsy 1 within each group.
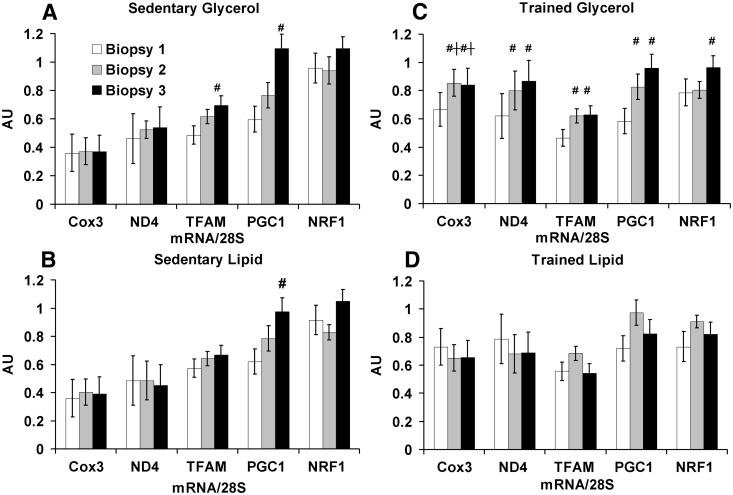
Fig. 4.
Mitochondrial mRNA alterations during the 6-h hyperinsulinemic-euglycemic clamp. The mitochondrial mRNA response to hyperinsulinemia differed between the groups. A, Sedentary glycerol (n = 7); B, sedentary lipid (n = 7); C, trained glycerol (n = 7); D, trained lipid (n = 7). All mRNA was quantitated as arbitrary units (AU) normalized to 28S ribosomal RNA. Values are shown as means ± se. †, P < 0.05 for the paired comparison between trained and sedentary groups with the same infusion. #, P < 0.05 for the paired comparison with biopsy 1 within each group.
Results
Baseline characteristics
Trained and sedentary participants were matched on age, gender, and BMI. In the trained group, nine participants had completed a recent marathon (within 1 yr), of which five had completed a marathon within the past month. Trained participants had a higher FFM, VO2 max, serum triglyceride concentration, mtDNA copy number (ND4/28S), insulin sensitivity (GIR from the 3-h hyperinsulinemic-euglycemic clamp at visit 2), and COX3 mRNA (Table 1) compared with sedentary participants. After adjusting for percentage body fat, only GIR remained significantly different between the trained and sedentary groups. Baseline VO2 max positively correlated with GIR (r = 0.394; P = 0.04), ND1 mtDNA (r = 0.445; P = 0.02), ND4 mtDNA (r = 0.540; P = 0.004), and COX3 mRNA (r = 0.41; P = 0.04).
Glucose, insulin, and FFA levels
During visit 3, there were no significant differences in the plasma glucose levels between the lipid and glycerol groups (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). In the trained group, the insulin level was lower in the trained lipid group at certain time points (120 and 300 min) compared with the trained glycerol group, and the insulin level trajectory was significantly different between the groups (P = 0.04) (Supplemental Fig. 1B). After 30 min of infusion, serum FFA was significantly higher (P = 0.04) in the lipid group (0.2 mEq/liter) than the glycerol group (0.07 mEq/liter) (P < 0.001). During the last hour of the lipid infusion, the serum FFA remained stable (0.6–0.7 mEq/liter) and significantly higher than the serum FFA with the glycerol infusion (∼0.0 mEq/liter; P < 0.01) (Supplemental Fig. 1C).
Effect of lipid/glycerol infusion on insulin sensitivity
With lipid infusion, both the trained and sedentary groups reduced GIR (P < 0.01) relative to their glycerol control (Fig. 2, A and B; −46.5% in sedentary group, −47.7% in trained group). For both activity groups, a significant divergence in GIR trajectory was observed between the lipid infusion and the glycerol infusion (P < 0.001). In the trained group, the divergence in GIR between the lipid and glycerol groups became significant earlier (120 vs. 240 min) compared with the sedentary group. The lipid vs. glycerol difference in the GIR AUC was comparable between the trained group (−28.4%) and the sedentary group (−27.8%) (Fig. 2C).
Insulin signaling
At biopsy 1, before initiation of any infusions, the trained and sedentary individuals had comparable levels of Ser 636/639-pIRS1/IRS1 and Ser 473-pAKT/AKT (Table 1 and Fig. 3, A–D). Because of the profound decrease in GIR with lipid infusion by the end of the hyperinsulinemic-euglycemic clamp, insulin signaling was measured at biopsy 3 (360 min) to examine whether the change in GIR was associated with alterations in insulin signaling. Only the trained glycerol group increased Ser 636/639-pIRS1/IRS1 at biopsy 3 (Fig. 3C) compared with biopsy 1 (P < 0.01). In all groups, there was no decline in Ser 473-pAKT/AKT at biopsy 3 compared with biopsy 1 (Fig. 3D).
Mitochondrial measures
At baseline, pAMPK/AMPK was comparable (Table 1) between the trained and sedentary groups. At the conclusion of the hyperinsulinemic-euglycemic clamp, pAMPK/AMPK was significantly altered in biopsy 3 relative to biopsy 1 only in the sedentary glycerol group (fold change 0.62: P = 0.01). This decline in the sedentary glycerol group was significant compared with the trained glycerol group (fold change 1.57: P < 0.01 compared with the sedentary glycerol group, P = 0.08 compared with biopsy 1) and the sedentary lipid group (fold change 1.52: P = 0.02 compared with the sedentary glycerol group, P = 0.37 compared with biopsy 1).
Skeletal muscle mitochondrial mRNA expression was measured in all three biopsies (Fig. 4). Relative to biopsy 1, the trained glycerol group had a significant increase in mRNA expression of COX3 (biopsy 2, biopsy 3), ND4 (biopsy 2, biopsy 3), PGC1a (biopsy 2, biopsy 3), TFAM (biopsy 2, biopsy 3), and NRF1 (biopsy 3) in response to the hyperinsulinemic-euglycemic clamp, none of which were observed in the trained lipid group. In contrast, the sedentary lipid group (PGC1a at biopsy 3) and the sedentary glycerol group (PGC1a and TFAM at biopsy 3) increased mRNA expression relative to biopsy 1 during the hyperinsulinemic-euglycemic clamp. When activity groups were compared, the trained glycerol group had a marked mitochondrial mRNA response to the hyperinsulinemic-euglycemic clamp that was not observed with lipid infusion; this effect was not observed in the sedentary group.
Discussion
Acute FFA elevation within the physiological range produced similar declines in insulin sensitivity in lean trained humans and lean sedentary humans. The FFA-associated reduction in insulin sensitivity was not clearly associated with impaired skeletal muscle insulin signaling, although the mitochondrial mRNA response to hyperinsulinemia in trained humans was impaired by FFA elevation. These findings suggest that the incremental benefit of training on insulin sensitivity may be associated with alterations in skeletal muscle mitochondrial mRNA expression, which is sensitive to FFA elevation.
Our observations confirm and extend those made by others. We demonstrate that a physiologically relevant FFA elevation (16) in trained humans can impair insulin sensitivity similar to a supraphysiological FFA elevation (14). By using a glycerol control, this study directly examined the metabolic effects of FFA elevation in trained humans, unlike previous work in trained participants that lacked such a control (12, 13).
In response to FFA elevation, the trained group had a comparable, if not more profound decline in GIR compared with the sedentary group. The trained group was more insulin sensitive than the sedentary group as documented by the GIR from visit 2 and by the GIR (150–180 min) from visit 3 with the glycerol infusion. When considering the equivalent time period between the hyperinsulinemic-euglycemic clamp at visit 2 (150–180 min) and visit 3 (150–180 min), the GIR decline of the trained lipid group was significant compared with the trained glycerol control (−36.1%; P = 0.01); in contrast, the GIR decline in the sedentary lipid group was not significant compared with the sedentary glycerol group (P = 0.21). These findings suggest that acute FFA elevation may reduce insulin sensitivity earlier in trained participants compared with sedentary participants. Nevertheless, training did not modify the difference in GIR at the end of the 6-h hyperinsulinemic-euglycemic clamp or the decline in AUC of GIR between the lipid and glycerol groups. This apparent lack of difference over the prolonged hyperinsulinemic-euglycemic clamp may be related to the GIR temporal pattern with glycerol infusion. In this study, the sedentary glycerol group gradually increased GIR over time compared with the stable GIR observed in the trained glycerol group. This is consistent with the previous observation that a variable, but gradual increase in GIR occurs during a prolonged hyperinsulinemic-euglycemic clamp, particularly in participants who are less insulin sensitive (24). The observed response of trained and sedentary participants to the lipid or glycerol infusion may be an adaptive response of muscle to available fuel, with trained participants being more readily adaptive than sedentary participants. When only glucose is available, as exemplified by hyperinsulinemic-euglycemic clamp, the muscle will use glucose. When fat and glucose are available, as exemplified by a lipid infusion during a hyperinsulinemic-euglycemic clamp, the muscle will use both fat and glucose, thereby reducing glucose uptake as demonstrated by reduced insulin sensitivity.
Regardless of training status or administered infusion, Ser 473 pAKT/AKT was not impaired after infusion despite the profound decline in insulin sensitivity from acute FFA elevation. These findings are consistent with other studies measuring insulin signaling at baseline and at the end of the insulin clamp (25–27), which do not show disruption in traditional insulin signaling, specifically the Ser 473 pAKT response. These findings do not exclude the possibility that early alterations in insulin signaling, as observed by measuring insulin signaling before and 30 min after insulin stimulation (16, 28), may contribute to the reduction in insulin sensitivity, although this early decline has not been consistently observed (26).
This study makes the novel observation that the trained glycerol group had enhanced AMPK phosphorylation, higher mitochondrial mRNA expression, and higher GIR relative to the sedentary glycerol group during the prolonged hyperinsulinemic-euglycemic clamp, suggesting that training-associated improvements in insulin sensitivity may be related to enhanced mitochondrial function. We acknowledge that mitochondrial mRNA expression as an approximation of mitochondrial function may be a limitation. Yet, it has been previously shown that hyperinsulinemia enhances skeletal muscle mitochondrial mRNA expression, mitochondrial protein synthesis, and mitochondrial ATP production (29).
In terms of potential clinical relevance, it has been previously shown that consumption of a single high-fat meal (1125 kcal, 30% carbohydrate, 61% fat, 9% protein) increased plasma FFA 4 h later to 0.8 mmol/liter, which was similar to the FFA level achieved by lipid infusion in this study. In contrast, a single high-carbohydrate meal (1115 kcal, 79% carbohydrate, 10% fat, 11% protein) increased plasma FFA 4 h later to only 0.2 mmol/liter (30). If orally ingested fat is presumed to only elevate FFA, then high fat intake may acutely reduce insulin sensitivity even in trained participants. However, a high-fat diet likely modulates cholecystokinin, peptide YY, glucagon-like peptide 1, and ghrelin secretion in addition to acutely elevating FFA (31). By bypassing the gut, the lipid infusion model of acute FFA elevation is a limited representation of the physiological effect of oral fat ingestion; it remains likely that the muscular response to acute FFA elevation from oral fat ingestion is more complicated than currently assessed.
Strengths and limitations
There are several strengths to the paper. One strength is the matching of healthy lean individuals by age, gender, and BMI, allowing multiple pairwise comparisons between the trained/sedentary and lipid/glycerol groups. Another methodological strength of this paper is the use of glycerol as a control infusion. Due to its triglyceride content, the lipid infusion is expected to increase plasma FFA and glycerol levels. Given that glycerol exposure has been previously shown to enhance endogenous glucose production (32), this study limited the effect of lipid infusion to FFA elevation by using a glycerol control matched to the glycerol content of the lipid infusion.
Several limitations are acknowledged. Although the design of the study could be enhanced by a randomized crossover design, the study would be limited by the number of participants willing to have multiple biopsies (three biopsies) over multiple visits (two visits) as well as the ability to maintain their activity program between the biopsy visits. Another limitation was performing the study at rest, at least 48 h from active exercise, which limits the metabolic effect of current or recent exercise. The rest period was defined to avoid the effect of recent exercise on insulin sensitivity and lipid metabolism (33–36). Although the benefit of endurance exercise may decline with detraining, the metabolic effect of training appears to persist over several weeks. Previously, it has been shown that trained humans subjected to 10 d of detraining did not change body composition or VO2 max but had a slight decline in skeletal muscle glucose transporter 4 protein and citrate synthase activity, which still remained higher than untrained controls (37). Another limitation of the study is the reliance on self-report of endurance training instead of a training program (3), yet the cross-sectional design was intentional due to the desire to compare sedentary and endurance-trained individuals (i.e. marathon runners), which would be difficult to achieve with a training program. Lastly, this study did not assess the slow and fast twitch fibers from the biopsy samples, which may be important because the mitochondrial content may vary by fiber type (9) and fiber type may change with training (3, 9).
Conclusion
The incremental benefit of endurance training on insulin sensitivity in lean humans may be associated with alterations in the mitochondrial mRNA response to hyperinsulinemia, which is readily susceptible to the metabolic consequences of acute FFA elevation within the physiological range.
Supplementary Material
Acknowledgments
We are grateful for the assistance of the University of Minnesota Clinical Translation Science Institute; outstanding nursing support by Michelle Snyder, R.N. (Clinical and Translational Science Institute, University of Minnesota) and Michael Mech, R.N. (Department of Medicine, University of Minnesota); the skillful review and editing by Dr. Anne Marie Weber-Main (Department of Medicine, University of Minnesota); and the guidance of Dr. LaDora Thompson and Windy Torgerud (Department of Physical Medicine and Rehabilitation, University of Minnesota) with the muscle biopsies. Special thanks are expressed to the study participants.
This work was supported by the National Center for Research Resources (5K12RR023247-02, M01-RR-00400), the Minnesota Medical Foundation, the Pennock Professorship, and the Minnesota Obesity Center (National Institutes of Health Grant DK050456).
L.S.C. developed the idea, analyzed the data, contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript. E.R.S. contributed to the discussion, and reviewed/edited the manuscript. L.E.E. directed the analysis of the data, contributed to the discussion, and reviewed/edited the manuscript. M.T.M. produced the insulin signaling data, contributed to the discussion, and reviewed/edited the manuscript. J.M.S. produced the mitochondrial data, contributed to the discussion, and reviewed/edited the manuscript. K.S.N. contributed to the discussion, and reviewed/edited the manuscript. D.G.M. analyzed the data, contributed to the discussion, and reviewed/edited the manuscript. There are no conflicts of interest reported by any of the authors.
L.S.C. will be the guarantor of the manuscript and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Part of these data were previously published in abstract form in the 2011 American Diabetes Association Meeting Abstract Book (457-PP, 760-P).
www.Clinicaltrials.gov Identifier: NCT00786487.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMPK
- AMP kinase
- AUC
- area under the curve
- BMI
- body mass index
- COX3
- cytochrome C oxidase subunit 3
- FFA
- free fatty acid
- FFM
- fat free mass
- GIR
- glucose infusion rate
- IMCL
- intramyocellular lipid
- IRS1
- insulin receptor substrate
- mtDNA
- mitochondrial DNA
- ND1
- NADH dehydrogenase subunit 1
- ND4
- NADH dehydrogenase subunit 4
- NRF1
- nuclear respiratory factor 1
- pAMPK
- phosphorylated AMPKα (Thr172)
- PGC-1a
- peroxisome proliferator-activated receptor γ coactivator 1α
- pIRS1
- phosphorylated IRS1
- TFAM
- mitochondrial transcription factor A
- VO2 max
- maximal oxygen consumption.
References
- 1. Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. 1990. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 322:223–228 [DOI] [PubMed] [Google Scholar]
- 2. Raji A, Seely EW, Arky RA, Simonson DC. 2001. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab 86:5366–5371 [DOI] [PubMed] [Google Scholar]
- 3. Dubé JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. 2008. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab 294:E882–E888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. 1994. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17:961–969 [DOI] [PubMed] [Google Scholar]
- 5. Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS., Jr 1991. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med 325:147–152 [DOI] [PubMed] [Google Scholar]
- 6. Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. 1999. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia [Erratum (1999) 42:386] 42:113–116 [DOI] [PubMed] [Google Scholar]
- 7. Itani SI, Ruderman NB, Schmieder F, Boden G. 2002. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51:2005–2011 [DOI] [PubMed] [Google Scholar]
- 8. Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. 2007. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Investig 117:1679–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goodpaster BH, He J, Watkins S, Kelley DE. 2001. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86:5755–5761 [DOI] [PubMed] [Google Scholar]
- 10. Holloszy JO, Coyle EF. 1984. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56:831–838 [DOI] [PubMed] [Google Scholar]
- 11. Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. 2003. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52:1888–1896 [DOI] [PubMed] [Google Scholar]
- 12. Matzinger O, Schneiter P, Tappy L. 2002. Effects of fatty acids on exercise plus insulin-induced glucose utilization in trained and sedentary subjects. Am J Physiol Endocrinol Metab 282:E125–E131 [DOI] [PubMed] [Google Scholar]
- 13. Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. 2009. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol 587:4949–4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boden G, Chen XH. 1995. Effects of fat on glucose-uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Investig 96:1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, Coleman E, Smith C. 1991. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Investig 88:960–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R, Defronzo RA, Cusi K. 2005. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes 54:1640–1648 [DOI] [PubMed] [Google Scholar]
- 17. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. 2003. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35:1381–1395 [DOI] [PubMed] [Google Scholar]
- 18. Block G, Gillespie C, Rosenbaum EH, Jenson C. 2000. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med 18:284–288 [DOI] [PubMed] [Google Scholar]
- 19. Cardinal BJ, Esters J, Cardinal MK. 1996. Evaluation of the revised physical activity readiness questionnaire in older adults. Med Sci Sports Exerc 28:468–472 [DOI] [PubMed] [Google Scholar]
- 20. Nair KS, Halliday D, Griggs RC. 1988. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol 254:E208–E213 [DOI] [PubMed] [Google Scholar]
- 21. Moran A, Jacobs DR, Jr, Steinberger J, Hong CP, Prineas R, Luepker R, Sinaiko AR. 1999. Insulin resistance during puberty. Results from clamp studies in 357 children. Diabetes 48:2039–2044 [DOI] [PubMed] [Google Scholar]
- 22. Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. 2008. Endurance exercise as a countermeasure for aging. Diabetes 57:2933–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. 2005. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102:5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soop M, Nygren J, Brismar K, Thorell A, Ljungqvist O. 2000. The hyperinsulinaemic-euglycaemic glucose clamp: reproducibility and metabolic effects of prolonged insulin infusion in healthy subjects. Clin Sci 98:367–374 [DOI] [PubMed] [Google Scholar]
- 25. Kruszynska YT, Worrall DS, Ofrecio J, Frias JP, Macaraeg G, Olefsky JM. 2002. Fatty acid-induced insulin resistance: decreased muscle PI3K activation but unchanged Akt phosphorylation. J Clin Endocrinol Metab 87:226–234 [DOI] [PubMed] [Google Scholar]
- 26. Høeg LD, Sjøberg KA, Jeppesen J, Jensen TE, Frøsig C, Birk JB, Bisiani B, Hiscock N, Pilegaard H, Wojtaszewski JF, Richter EA, Kiens B. 2011. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes 60:64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsintzas K, Chokkalingam K, Jewell K, Norton L, Macdonald IA, Constantin-Teodosiu D. 2007. Elevated free fatty acids attenuate the insulin-induced suppression of PDK4 gene expression in human skeletal muscle: potential role of intramuscular long-chain acyl-coenzyme A. J Clin Endocrinol Metab 92:3967–3972 [DOI] [PubMed] [Google Scholar]
- 28. Kashyap SR, Belfort R, Berria R, Suraamornkul S, Pratipranawatr T, Finlayson J, Barrentine A, Bajaj M, Mandarino L, DeFronzo R, Cusi K. 2004. Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Am J Physiol Endocrinol Metab 287:E537–E546 [DOI] [PubMed] [Google Scholar]
- 29. Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. 2003. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100:7996–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okano G, Sato Y, Takumi Y, Sugawara M. 1996. Effect of 4 h preexercise high carbohydrate and high fat meal ingestion on endurance performance and metabolism. Int J Sports Med 17:530–534 [DOI] [PubMed] [Google Scholar]
- 31. Little TJ, Horowitz M, Feinle-Bisset C. 2007. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: implications for the pathophysiology of obesity. Am J Clin Nutr 86:531–541 [DOI] [PubMed] [Google Scholar]
- 32. Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. 1983. Effect of fatty acids on glucose production and utilization in man. J Clin Investig 72:1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brechtel K, Niess AM, Machann J, Rett K, Schick F, Claussen CD, Dickhuth HH, Haering HU, Jacob S. 2001. Utilisation of intramyocellular lipids (IMCLs) during exercise as assessed by proton magnetic resonance spectroscopy (H-1-MRS). Horm Metab Res 33:63–66 [DOI] [PubMed] [Google Scholar]
- 34. Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. 1996. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335:1357–1362 [DOI] [PubMed] [Google Scholar]
- 35. Schenk S, Horowitz JF. 2007. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Investig 117:1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dufaux B, Order U, Muller R, Hollmann W. 1986. Delayed effects of prolonged exercise on serum lipoproteins. Metabolism 35:105–109 [DOI] [PubMed] [Google Scholar]
- 37. McCoy M, Proietto J, Hargreves M. 1994. Effect of detraining on Glut-4 protein in human skeletal muscle. J Appl Physiol 77:1532–1536 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.