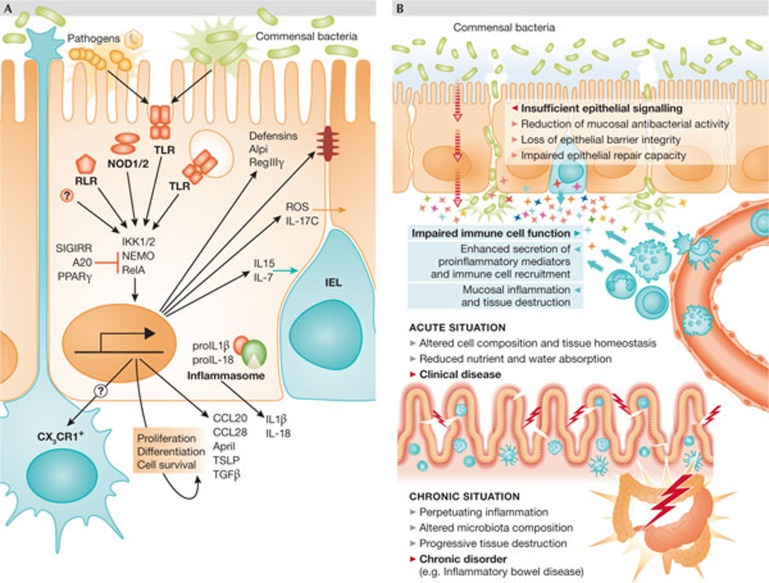
Figure 4.
Innate immune signalling in IECs. (A) Both pathogenic and commensal microorganisms stimulate innate immune receptors in IECs, such as TLRs, NLRs and RLRs. Gene expression is induced among other pathways through IKK1 and 2, NEMO and RelA/p65. Inflammasome activation leads to pro-IL-1β and pro-IL-18 cleavage by caspase 1, resulting in IL-1β and IL-18 secretion. The functional relevance of the inflammasome in IECs has not formally been shown. Innate immune signalling in IECs is also controlled by negative regulatory factors, such as SIGIRR/TIR8, A20 and PPARγ. IEC stimulation leads to the secretion of antimicrobial effectors, such as defensins and RegIIIγ, as well as the immunomodulatory Alpi, reinforces tight junctions and drives intraepithelial communication through the production of ROS and IL-17C. In addition, it facilitates cross-talk to professional immune cells of the lamina propria, leading to the recruitment of IELs through the secretion of IL-15 and IL-7, and stimulating CX3CR1+ non-migratory phagocytes to sample the luminal content. Finally, epithelial cell differentiation, proliferation and survival is influenced by the secretion of a variety of soluble mediators, such as CCL20, CCL28, April, TSLP and TGF-β is stimulated. (B) When the intestinal epithelial barrier loses integrity, commensal bacteria can translocate to the subepithelial tissue, inducing the secretion of proinflammatory mediators and leukocyte recruitment. This, in turn, induces organ dysfunction, reduced nutrient and water absorption, and can lead to mucosal inflammation and clinical disease. If the situation becomes chronic, it can impair wound healing and contribute to the development of disorders such as inflammatory bowel disease.