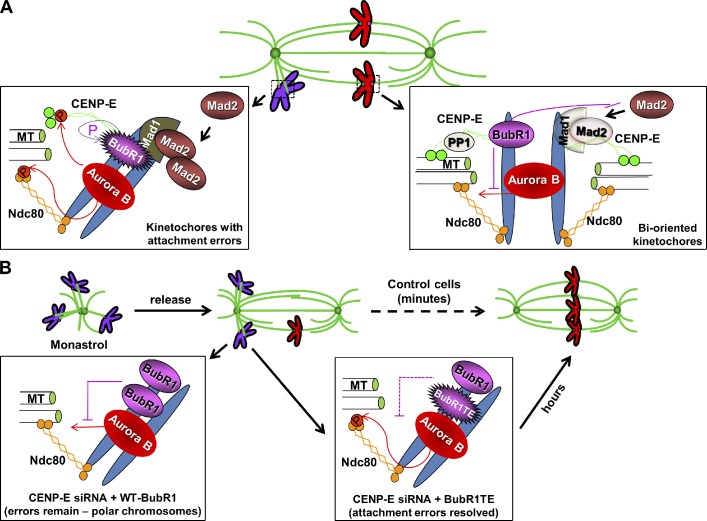
Figure 7.
A model for the role of CENP-E–dependent BubR1 autophosphorylation at the kinetochore. (A) Incorrect kinetochore–microtubule attachments (purple chromosomes) trigger Aurora B–mediated phosphorylation of the KMN network (represented by the Ndc80 complex in the cartoon) and CENP-E, resulting in destabilization of kinetochore microtubules. At unattached kinetochores, CENP-E stimulates BubR1 kinase activity and its autophosphorylation. Mad1 and Mad2 are also recruited onto unattached kinetochore to generate mitotic checkpoint signaling. At attached bioriented kinetochores (red chromosomes), spindle microtubule capture by CENP-E silences BubR1 kinase activity and recruits Phosphatase 1 (PP1) to outer kinetochores. The nonphosphorylated form of BubR1 reduces Aurora B–mediated Ndc80 phosphorylation and Mad1–Mad2 association at kinetochores. These coordinated events result in dephosphorylation of key components at the kinetochore–microtubule interface (e.g., Ndc80), leading to stable attachment upon biorientation and separation of the inner centromeric Aurora B from outer kinetochore substrates, as well as silencing the mitotic checkpoint signaling. (B) During the formation of the bipolar spindle upon release from monastrol treatment, a large number of attachment errors needs to be corrected. In the absence of CENP-E, the nonphosphorylated form of endogenous BubR1 resides on incorrectly attached kinetochores, which reduces Aurora B–mediated Ndc80 phosphorylation, leading to the persistent existence of polar chromosomes. Expressing a phosphomimetic BubR1 mutant to replace some of the nonphosphorylated endogenous BubR1 at kinetochores helps to correct misattachments, although it takes a much longer time for those cells to achieve metaphase chromosome alignment. MT, microtubule; P, phosphorylated.