Figure 7. CpdR-Competent PdeA Subunit Supports Proper Degradation of the Entire Dimer.
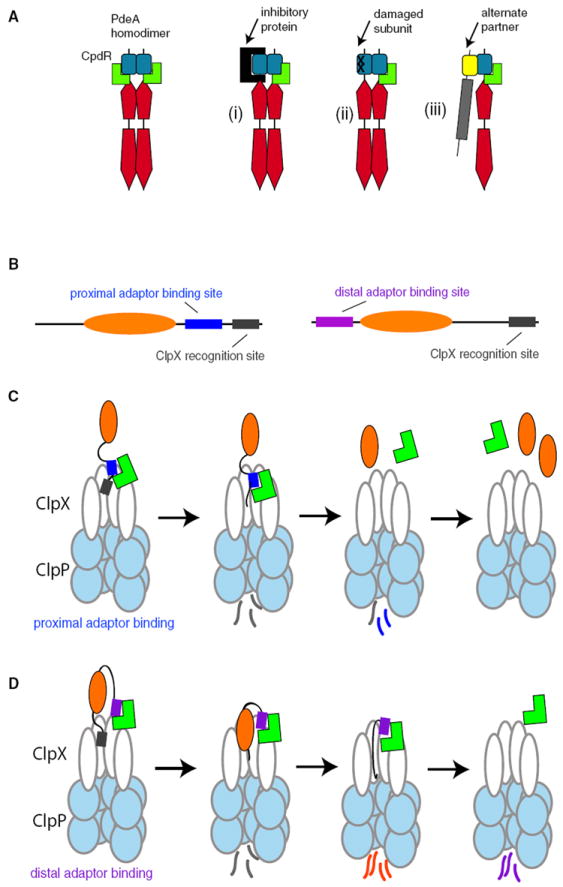
(A) Dimerization of PdeA via the PAS domain may improve degradation in vivo if one subunit is prevented from CpdR binding by inhibitory protein binding (i) or damage to the subunit (ii). Alternatively, PdeA may facilitate CpdR-dependent degradation by bridging CpdR and an alternate binding partner (iii).
(B) Cartoon illustrating organization of adaptor-binding (blue or purple) versus protease-recognition sites (gray) for different substrate architectures.
(C) Binding of adaptor (green) to sites proximal to or overlapping with protease recognition sites in a substrate improves recognition. Because both the recognition site and the adaptor site are the first to be degraded, any release of adaptor would result in a buildup of partially processed substrates.
(D) Adaptor binding to sites distal to the recognition tag promotes tight association between the protease and substrate even after proteolysis has begun, potentially leading to substrate reengagement and complete degradation.
