Abstract
Enlarging or new lesions frequently appear on magnetic resonance imaging (mri) after concurrent administration of radiation therapy and temozolomide in glioblastoma multiforme (gbm) patients. However, in nearly half such cases, the observed radiologic changes are not due to true disease progression, but instead are a result of a post-radiation inflammatory state called “pseudoprogression.” Retrospective studies have reported that neurologic deterioration at the time of the post-chemoradiotherapy mri is found more commonly in patients with true disease progression. We report a gbm patient with both radiologic progression on the post-chemoradiotherapy mri and concomitant neurologic deterioration, and we caution against incorporating clinical deterioration into the management schema of patients with possible pseudoprogression.
Keywords: Pseudoprogression, disease progression, neurologic deterioration, glioblastoma multiforme, mri, chemotherapy, radiation therapy, temozolomide
1. INTRODUCTION
The current standard for postoperative treatment of glioblastoma multiforme (gbm) is a combination of temozolomide given concurrently with radiation therapy (rt), followed by adjuvant temozolomide alone1. After concurrent chemoradiotherapy, it is not unusual to see an enlarging lesion on the first post-chemoradiotherapy magnetic resonance imaging (mri)2–6. In approximately 50% of cases, the “progressing” lesions stabilize or diminish with additional cycles of temozolomide4,6. This phenomenon is called “pseudoprogression” because its appearance mimics disease progression on mri3. The ability to distinguish between true progression and pseudoprogression is therefore critical, because true progressors require a change in treatment, but pseudoprogressors should continue with adjuvant temozolomide.
Currently, there are no definitive radiologic criteria to differentiate between true progression and pseudoprogression. The response criteria for brain tumours developed by the Response Assessment in Neuro-Oncology Working Group state that apparent radiologic progression can be considered true progression within 12 weeks of completion of chemoradiotherapy only if new lesions have appeared outside the radiation field or if pathology confirmation of progressive disease has been obtained7. Histopathology might assist in making the differentiation, but the analysis can be challenging, because specimens may contain viable tumour, necrosis, a mixed infiltrate of acute and chronic inflammatory cells, and edema8. Hence, given the high incidence of pseudoprogression and the absence of definitive radiologic criteria, it is impractical to subject all such patients to surgery, particularly if they are clinically stable. Therefore, the distinction between true progression and pseudoprogression is made retrospectively by comparing the first post-chemoradiotherapy mri with subsequent imaging. Canadian gbm treatment guidelines recommend that 3 cycles of adjuvant temozolomide be given before a decision is made about whether the initial imaging changes represent true disease progression or pseudoprogression9,10.
In the absence of definitive radiologic criteria or histopathologic diagnosis, an enlarging lesion within the radiation field on the first post-chemoradiotherapy mri presents a diagnostic dilemma to the oncologist. As a result, oncologists often look for other clues to help differentiate between true progression and pseudoprogression. One such example is the presence of neurologic deterioration at the time of the first post-chemoradiotherapy mri. Intuitively, it is logical that, in a patient with a radiologically enlarging lesion, worsening neurologic function is more likely to be associated with true disease progression. That association is supported by data from two retrospective studies2,6. In such a situation, an oncologist may therefore choose to treat the patient as a true progressor and change treatment.
Here, we report a case that demonstrates the pitfalls of using neurologic deterioration to differentiate between pseudoprogression and true disease progression in gbm patients who have completed chemoradiotherapy.
2. CASE DESCRIPTION
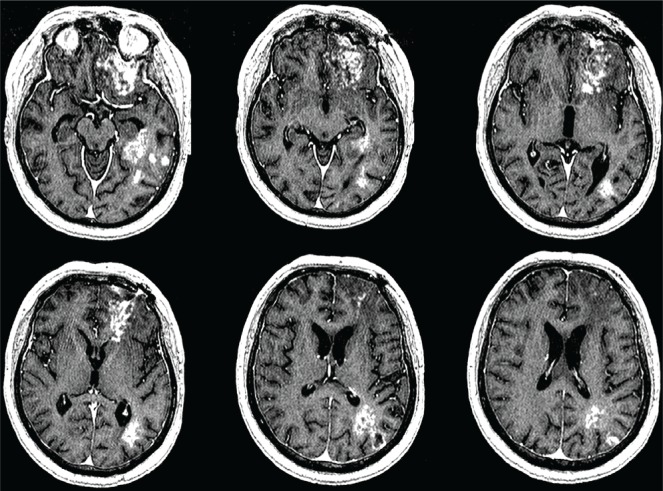
A 54-year-old man presented in March 2009 with progressive memory disturbance, diminished comprehension, and disorientation for 2 months. Brain mri showed multiple heterogeneously enhancing masses in the left cerebral hemisphere (basifrontal: 47×16×22 mm; medial temporal: 44×28×33 mm; parieto-occipital: 15×15×16 mm; Figure 1). He underwent a left frontal craniotomy and biopsy of the basifrontal mass on March 28, 2009.
FIGURE 1.
Preoperative magnetic resonance imaging of brain with contrast shows multiple heterogeneously enhancing lesions in the left basifrontal, medial temporal, and parieto-occipital regions.
The pathology was consistent with gbm and the O6-methylguanine methyltransferase (mgmt) promoter was methylated. Postoperatively, the patient received rt (3-dimensional conformal to a dose of 5040 cGy in 28 fractions) and chemotherapy (temozolomide 75 mg/m2 daily) concurrently for 6 weeks. Because of the extensive radiation volume (involving almost the entire left cerebral hemisphere), the dose was limited to minimize rt-associated toxicity. After a 4-week treatment-free period, the patient started the adjuvant phase of temozolomide (150–200 mg/m2 daily in a 5-in-28-days cycle).
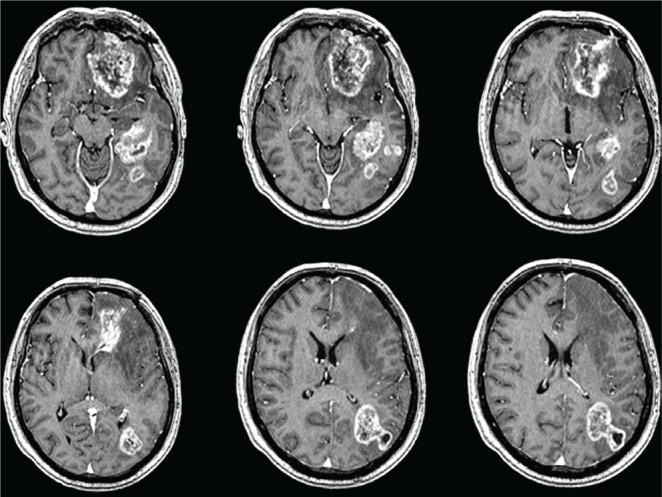
While on his first cycle of adjuvant temozolomide, the patient presented to the Neuro-oncology clinic with features of rapidly deteriorating memory (for both recent and remote events) and receptive aphasia. The first post-chemoradiotherapy mri showed a significant increase in the basifrontal (58×40×38 mm) and parieto-occipital (40×26×41 mm) lesions, with associated perilesional edema and mass effect. In addition, two new satellite lesions (within the 95% isodose line of radiation) were evident lateral to the medial temporal mass (Figure 2).
FIGURE 2.
Post chemoradiotherapy, the first magnetic resonance imaging of brain with contrast shows enlarged basifrontal and parieto-occipital lesions. Two new satellite lesions are seen lateral to the medial temporal mass.
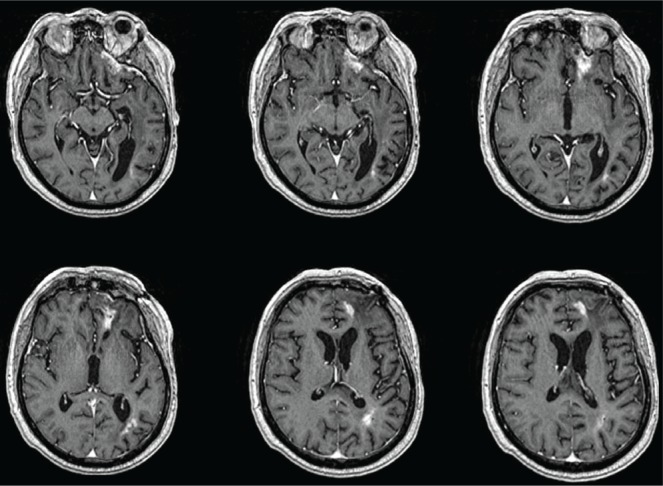
Dexamethasone was increased to 8 mg from 2 mg daily. A neurosurgical consultation was sought to decompress the lesions and obtain tissue for histopathology, but the procedure was not considered feasible. Because the patient had radiologic evidence of increased lesion size, new lesions within the rt treatment field, and new focal neurologic deficits, disease progression was considered to be the likely cause, in the absence of histologic confirmation. The chemotherapeutic regimen was altered to 4-weekly cycles of low-dose metronomic temozolomide (50 mg/m2 daily)11. The patient’s symptoms gradually improved over the next 2 weeks, and steroid tapering was started after a review of the next brain mri (Figure 3). Serial mri (every 2 months) continued while the patient remained on chemotherapy. During cycles 2, 9, and 23 of metronomic temozolomide (Figures 3–5 respectively), mri showed progressive reduction in the size of the lesions. The patient was still living at 30 months after surgery, when this report was submitted.
FIGURE 3.
During cycle 2 of metronomic temozolomide, magnetic resonance imaging of brain with contrast shows lesions decreased in size compared with those in the first imaging post chemoradiotherapy (Figure 2).
FIGURE 5.
During cycle 23 of metronomic temozolomide, magnetic resonance imaging of brain with contrast shows a further decrease in the size of the lesions compared with that see in imaging during cycle 9 (Figure 4).
FIGURE 4.
During cycle 9 of metronomic temozolomide, magnetic resonance imaging of brain with contrast shows lesions markedly decreased in size compared with that in earlier imaging (Figures 2 and 3).
3. DISCUSSION
This report describes a common clinical presentation in which a gbm patient treated with chemoradiotherapy presents with apparent radiologic and neurologic deterioration. In the absence of randomized controlled data, oncologists must decide whether such a patient is a true progressor or a pseudoprogressor. The incidence of pseudoprogression, as a proportion of all enlarging or new lesions seen on the first post-chemoradiotherapy mri, is reported to be 32%–64%2,4–6. Brandsma et al.3 suggested that pseudoprogression is the result of treatment (rt + temozolomide)–related local tissue reaction with an inflammatory component, edema, and abnormal vessel permeability, which causes new or enlarged contrast enhancement on imaging3.
The Response Assessment in Neuro-Oncology Working Group recently developed new standardized response criteria for brain tumours7. According to those guidelines, true disease progression within 12 weeks of chemoradiotherapy completion (when pseudoprogression is most prevalent) can be diagnosed only if the lesions are outside the radiation field (beyond the high-dose region or the 80% isodose line) or if unequivocal evidence of viable tumour is found on histopathology. Current Canadian treatment guidelines also advocate waiting for 3 cycles of adjuvant therapy after chemoradiotherapy to determine if gbm is truly progressing9,10. However, the time interval (about 2 months) required to differentiate pseudoprogression from true progression is often distressing for both the patient and the oncologist. In the absence of tissue confirmation, a few parameters may distinguish between pseudoprogression and true progression. Some authors have postulated that mgmt promoter methylation status may help to differentiate the two. The significance of mgmt promoter methylation in glioblastoma and its association with improved survival was described by Hegi et al. in 200512. Retrospective data suggest that pseudoprogression may occur more commonly in mgmt promoter–methylated gbm tumours than in unmethylated tumours (p = 0.0002) and that it is associated with improved survival2. However, that observation has not been validated in a randomized study, and the ability to test for mgmt methylation status is not readily available in all centres.
As a result, physicians may be tempted to include clinical data such as neurologic deterioration to help differentiate pseudoprogressors from true progressors. Support for that approach can be found in a report by Taal et al.6, who observed that two thirds of patients with high-grade glioma experiencing neurologic deterioration were true progressors. Similarly, Brandes et al.2 reported neurologic deterioration after chemoradiotherapy in 55.6% of gbm patients with disease progression, but in only 34% of patients with pseudoprogression. Importantly, although the data are highly suggestive, neither of the foregoing reports advocates that neurologic deterioration be used to differentiate between disease progression and pseudoprogression. That said, in the absence of other parameters, physicians may choose to use neurologic deterioration to inform their treatment decision.
In the present case, faced with apparent radiologic progression and definite neurologic deterioration, we leaned toward a diagnosis of true disease progression. In retrospect, we now believe that the enlarged and new lesions on the first post-chemoradiotherapy mri (Figure 2) were indicative of pseudoprogression. That revised opinion is supported by the fact that the original tumour was mgmt promoter–methylated, which is associated with pseudoprogression and improved overall survival2. Furthermore, although the second-line metronomic temozolomide regimen is associated with an overall 6-month progression-free survival of about 24%11, almost no patients have survived more than 1 year on the regimen. Hence, it is unlikely that the second-line treatment regimen resulted in the prolonged survival and the dramatic radiologic response seen in the present case (Figures 3–5). Had the chemotherapeutic regimen not been altered, there is a strong possibility that the clinical and radiologic outcome would have been comparable to the patient’s current status.
The development of new neurologic symptoms coupled with adverse radiologic changes on the first post-chemoradiotherapy mri does not rule out the possibility of pseudoprogression. Until a definitive method of diagnosing either pseudoprogression or disease progression at the time of the first post-chemoradiotherapy mri is identified, we a dvocate that the decision to change therapy should be deferred until 3 cycles of adjuvant temozolomide have been completed.
4. ACKNOWLEDGMENT
The authors thank Dr. Gerald Lim, radiation oncologist, Tom Baker Cancer Centre, Calgary, Alberta, for his assistance with this manuscript.
5. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to report.
6. REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. on behalf of the European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Brandes AA, Franceschi E, Tosoni A, et al. mgmt promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–7. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 3.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–61. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 4.Roldán GB, Scott JN, McIntyre JB, et al. Population-based study of pseudoprogression after chemoradiotherapy in gbm. Can J Neurol Sci. 2009;36:617–22. doi: 10.1017/s0317167100008131. [DOI] [PubMed] [Google Scholar]
- 5.Sanghera P, Perry J, Sahgal A, et al. Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci. 2010;37:36–42. doi: 10.1017/s0317167100009628. [DOI] [PubMed] [Google Scholar]
- 6.Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405–10. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 7.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 8.Perry A, Schmidt RE. Cancer therapy-associated cns neuropathology: an update and review of the literature. Acta Neuropathol. 2006;111:197–212. doi: 10.1007/s00401-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 9.Mason WP, Maestro RD, Eisenstat D, et al. Canadian recommendations for the treatment of glioblastoma multiforme. Curr Oncol. 2007;14:110–17. doi: 10.3747/co.2007.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Easaw JC, Mason WP, Perry J, et al. Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol. 2011;18:e126–36. doi: 10.3747/co.v18i3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry JR, Bélanger K, Mason WP, et al. Phase ii trial of continuous dose-intense temozolomide in recurrent malignant glioma: rescue study. J Clin Oncol. 2010;28:2051–7. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 12.Hegi ME, Diserens AC, Gorlia T, et al. mgmt gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]