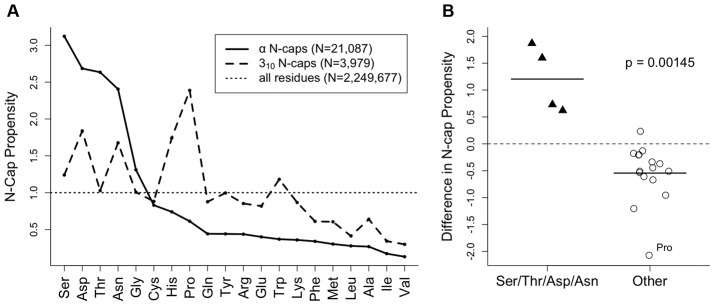
Figure 2. N-cap propensities vary by amino acid type.
(A) The 20 amino acid types are shown ranked according to their α-helix N-cap propensity (solid line), defined as the fraction of α-helix N-cap residues of the given amino acid type, divided by the fraction of general case residues of that amino acid type (dotted line). The correlation with the analogously defined 310-helix N-cap propensity (dashed line) is surprisingly weak, except for both slightly disfavoring hydrophobics. For example, Ser/Asp/Thr/Asn are the most common N-caps for α-helix but are not especially favored as N-caps for 310-helix. Some other hydrophobic amino acids like Ala/Ile/Val are uncommon as either type of N-cap. (B) The canonical α-helix N-caps Ser/Thr/Asp/Asn (triangles) are grouped separately from the other 16 amino acid types (circles); the two groups are compared based on the difference between α-helix N-cap propensity and 310-helix propensity. The horizontal dotted line at 0.0 indicates neither an increase nor a decrease in preference for α-helix N-caps instead of 310-helix N-caps. A one-tailed Mann-Whitney test shows with 95% confidence (p-value = 0.00145<α = 0.05) that Ser/Thr/Asp/Asn are statistically unique in terms of their specificity for α-helix N-caps.