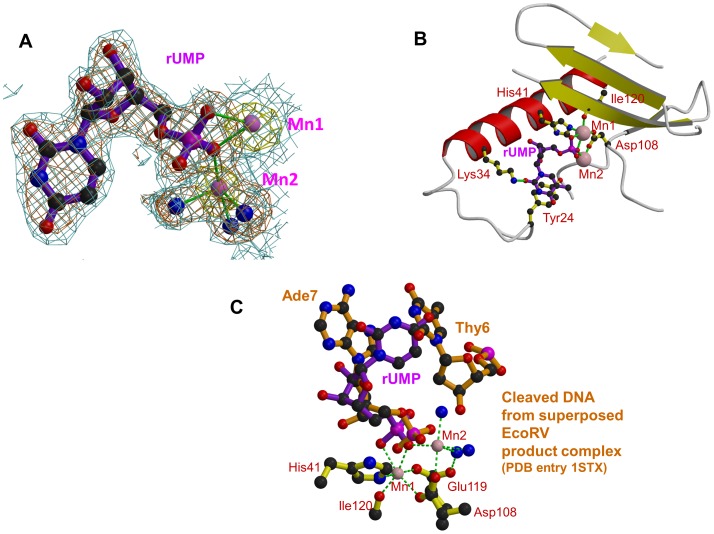
Figure 3. Binding of dTMP/rUMP in the active site of pH1N1 endonuclease.
A: Electron density for rUMP in pH1N1 PA endonuclease. Manganese ions are pink spheres, co-ordinating water molecule blue spheres and the ion co-ordination is shown with green lines. Blue contour: final 2Fo-Fc electron density at 1.0σ. Brown contour: Fo-Fc unbiased difference map at 2.8σ. Yellow contour: anomalous density at 4.0σ. B: Binding site of rUMP in the active site following the same scheme as in Figure 2. C: rUMP bound in the active site of pH1N1 PA (purple) with superposed DNA from product complex of EcoRV (brown, pdb entry 1STX). Active site residues (yellow), manganese ions (pink) and water molecules (blue) are for the rUMP structure. The position of the two DNA bases either side of the cleavage site in the EcoRV product complex is shown.