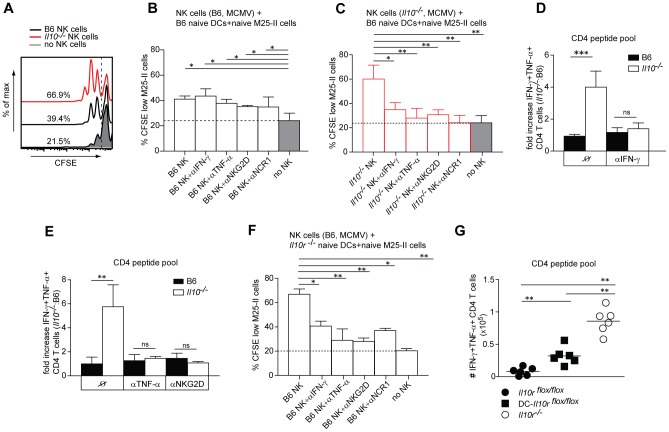
Figure 7. Unleashed NK/DC crosstalk promotes CD4 T cell priming in Il10 −/− mice.
B6 and Il10 −/− mice were infected with 5×106 PFU Δm157 MCMV. A–C,F) DX5+CD3− (NK) cells were isolated from B6 and Il10 −/− mice by MACS at day 3.5 p.i. Splenic DCs were isolated from naive B6 (A–C) mice or naive Il10r −/− mice (F) by enrichment for CD11c+ cells. MCMV-specific CD4 T cells were isolated by MACS from spleens of naive M25-II mice and labeled with CFSE. CD11c+ cells were loaded with M25 peptide and co-cultured with CFSE-labeled M25-II cells without (no NK) or with addition of DX5+CD3− (NK) cells isolated from B6 (A, B, F) or Il10 −/− (A, C) mice. As indicated, blocking antibodies for IFN-γ, TNF-α, NKG2D and NCR-1 were added to cultures. The frequencies of CFSElow M25 II cells are shown. (n = 3, data are representative of 3 independent experiments). Dotted lines indicate the mean level of M25-II CFSE dilution in cultures without NK cells (no NK). D, E) B6 and Il10 −/− mice were treated in vivo with αIFN-γ antibodies at days 3 and 4 p.i. (D); with αTNF-α and αNKG2D antibodies at days 0, 3, 4 p.i. (E). Lymphocytes from lungs of infected mice were isolated at day 5.5 p.i. and ex vivo restimulated with a pool of M14, m18, M25, M112, m139 and m142 peptides (CD4 peptide pool). Fold increase (D, E) of IFN-γ+ TNF-α+ peptide specific CD4 cells between Il10 −/− and B6 mice is shown (n = 3, error bars indicates standard deviation, data are representative for 3 experiments). G) DC- Il10r flox/flox, Il10r flox/flox and Il10r −/− mice were infected with 5×106 PFU Δm157 MCMV. Lymphocytes from lungs were restimulated with the CD4 peptide pool. Total numbers of IFN-γ+ TNF-α+ peptide-specific CD4 cells are shown. (n = 3, data are pooled from 2 independent experiments). Statistical analysis was performed by 2-tailed unpaired student's t-test (* p<0.05, ** p<0.01, *** p<0.001).