Figure 2.
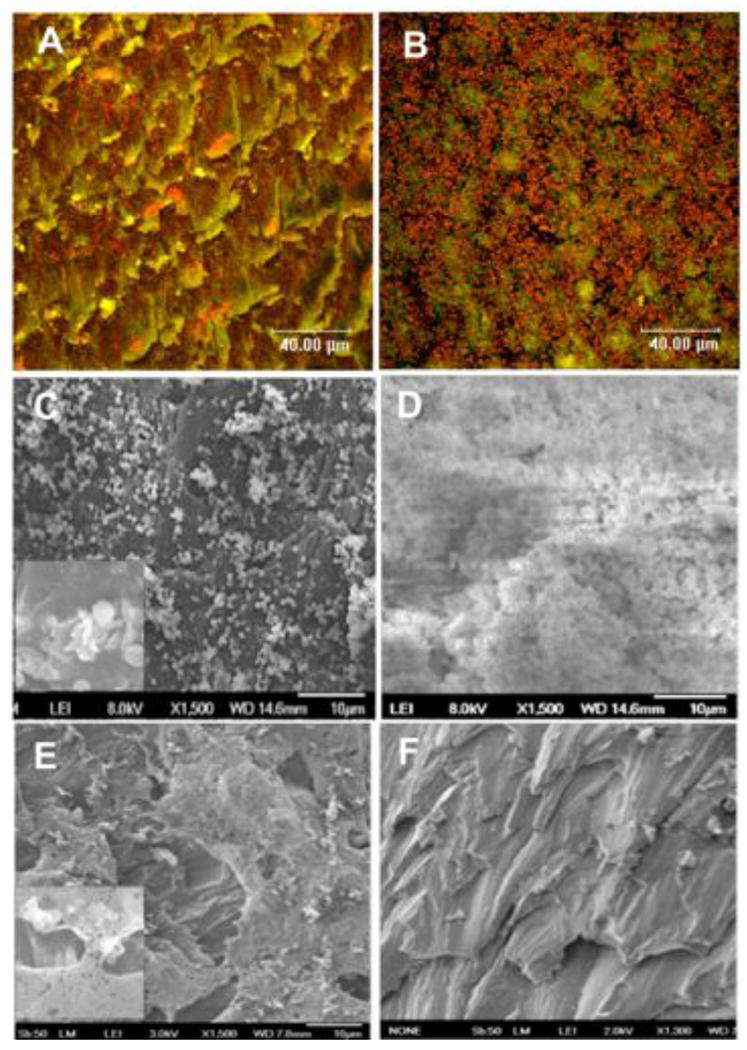
(A and B) Confocal laser scanning micrographs of S. aureus derived from an eye canniculus infection (MEEI-IB013) after 20 h of static culture on a disc in vitro. S. aureus grown on HMPEI-PMMA discs shows few colonies or small microcolonies (A), while the bacteria grown on control PMMA discs forms thick, confluent biofilm structures (B). Bacteria appear orange (1 micron in size) (acridine orange stain; three-channel combination of 488, 568, and 633 nm) (63X). (C, D) Scanning electron micrographs of S. aureus derived from a keratitis infection (MEEI-IB001) after 6 days of continuous static growth in vitro. S. aureus grown on HMPEI-PMMA discs shows single or small microcolonies with lysis of bacterial walls (1500X); inset shows higher magnification (5000X) of bacterial cell lysis (C). Confluent, mature bacterial biofilms are seen on control PMMA discs (1500X) (D). (E) SEM of laboratory-derived hyperbiofilm forming S. aureus strain (MN8m) after 6 days of continuous static growth on HMPEI-PMMA discs in vitro. S. aureus bacterial colonies are seen suspended on bacterial-derived extrapolysaccharide matrix, while no bacteria are seen on the HMPEI-PMMA disc below (1500X). Inset shows a higher magnification (4000X) of bacterial colonies suspended over HMPEI-PMMA material with lysis of bacterial walls on contact with it. (F) SEM of a no-bacteria control of HMPEI-PMMA discs. The rough cutting of PMMA disc is unaltered by covalent attachment of HMPEI to it (1300X).
