Figure 9.
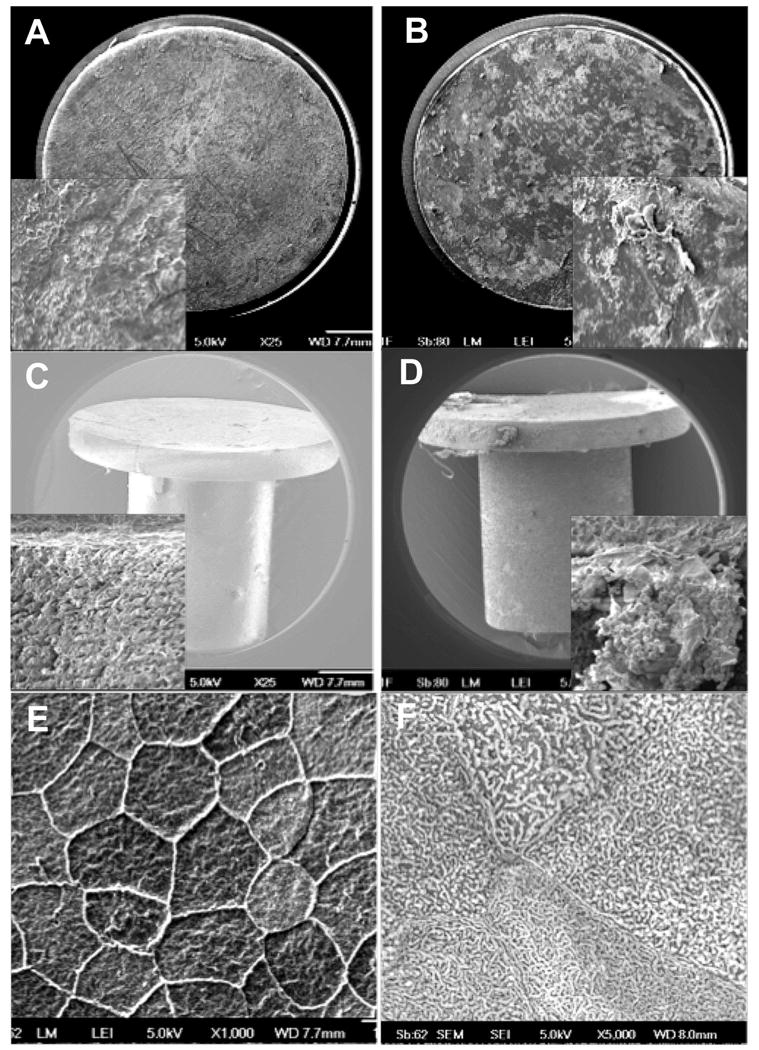
Representative scanning electron micrographs of HMPEI-titanium disc (A) compared to a control titanium disc (B) extracted from the corneal stroma after 61 days in the rabbit in vivo. (A) Cells attached to the HMPEI-Ti discs appear smoothly confluent (25X). A higher magnification inset shows a dense “basketweave” of epithelial cells on the HMPEI-Ti disc (500X). (B) Cells attached to the control Ti discs demonstrate a less contiguous and more disorganized covering on the uncoated disc (25X). The higher magnification inset shows rolled up edges of discontinuous stromal cells on the control Ti disc (500X). SEMs of (C) HMPEI-PMMA KPro-FP compared to (D) control PMMA KPro-FP surgically extracted at post-operative day 105. (C) Micrograph shows denser, more compact stromal cells adherent to HMPEI-PMMA KPro-FP (25X). A higher magnification inset shows a dense “basketweave” of epithelial cells (500X). (D) There is looser, discontinuous cellular material on the PMMA KPro-FP (25X). The higher magnification inset shows red blood cells in a capillary loosely attached to stromal cells on the front piece (500X). SEMS of (E) HMPEI-coated netilicon and (F) HMPEI-methafilcon contact lenses at 22 days after placement on the rabbit cornea. (E) Micrograph shows organized, smoothly confluent normal mosaic of light, medium, and dark epithelial cells adhering to the HMPEI-coated netilicon contact lens (1000X). (F) A higher magnification shows normal epithelial cell microplicae on the HMPEI-coated methafilcon contact lens (5000X).
