Abstract
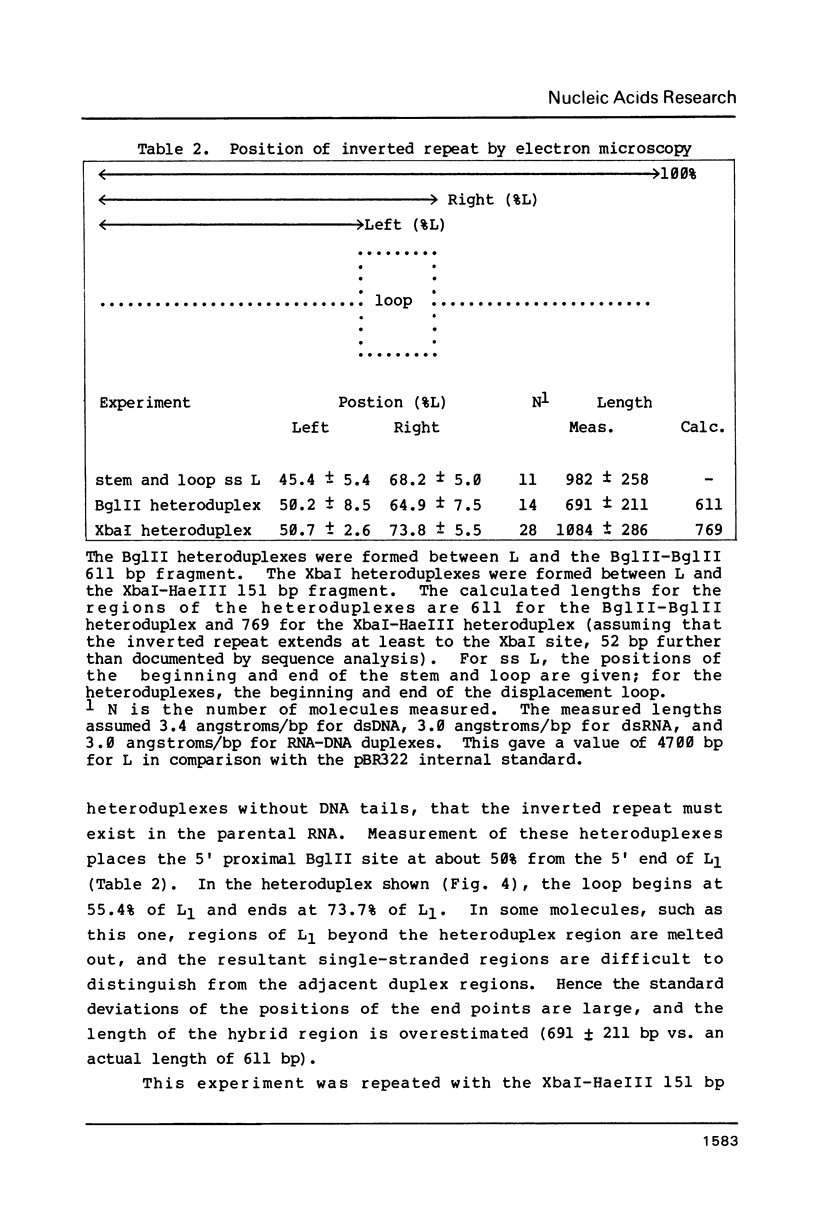
The Saccharomyces cerevisiae viruses are non-infectious double-stranded (ds) RNA viruses present in most laboratory strains of yeast. Their genome consists of one or more dsRNAs separately encapsidated in particles composed mainly of one polypeptide, which has a Mr of 88 kdaltons in the best-studied viral subtype. A large viral dsRNA (L1, of 4.7 kb) encodes the capsid polypeptide. We have determined the sequences of a number of cDNA clones homologous to portions of L1 and mapped them by a novel heteroduplex technique. Several of these clones originate from a region of L1 2.3-2.5 kb from the 5' end of the plus strand that contains stop codons in all three reading frames in the plus strand. We therefore suspect that the capsid polypeptide gene lies in the 5' 2.3-2.6 kb of the plus strand. One of the cloned cDNAs has an inverted repeat of 170 bp that appears to be present in its parental RNA. The inverted repeat in L1 is the longest known inverted repeat in a viral dsRNA and the only known non-terminal inverted repeat. It might serve the function of creating two mRNAs from one viral dsRNA.
Full text
PDF



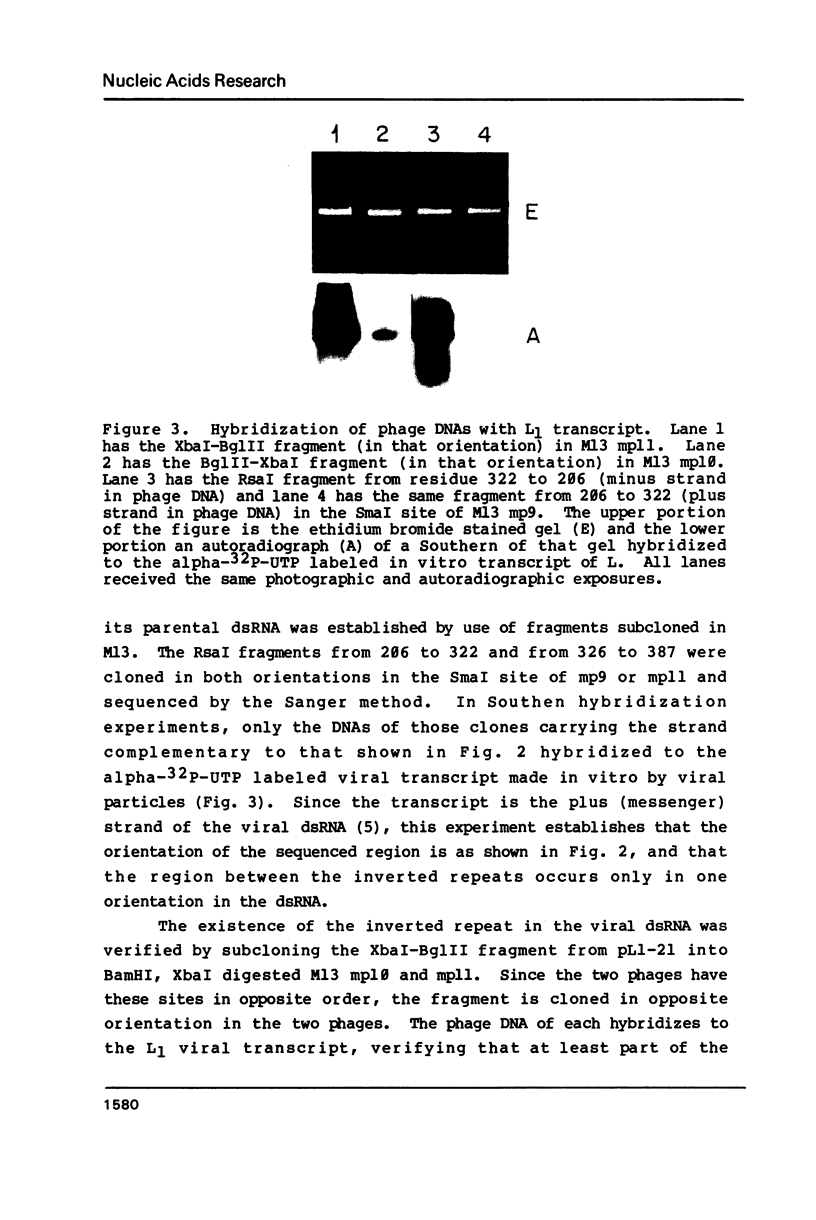










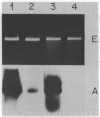
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bobek L. A., Bruenn J. A., Field L. J., Gross K. W. Cloning of cDNA to a yeast viral double-stranded RNA and comparison of three viral RNAs. Gene. 1982 Sep;19(2):225–230. doi: 10.1016/0378-1119(82)90010-5. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Burn V. E., Jayachandran S., Tipper D. J. Yeast killer dsRNA plasmids are transcribed in vivo to produce full and partial-length plus-stranded RNAs. Nucleic Acids Res. 1983 Feb 25;11(4):1077–1097. doi: 10.1093/nar/11.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Sturgeon J. A., Tipper D. J. Encapsidation of yeast killer double-stranded ribonucleic acids: dependence of M on L. J Bacteriol. 1980 Jul;143(1):463–470. doi: 10.1128/jb.143.1.463-470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Mattick J. S., Bellamy A. R. Serotype-specific glycoprotein of simian 11 rotavirus: coding assignment and gene sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3091–3095. doi: 10.1073/pnas.80.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Siegman L. J., Bellamy A. R., Atkinson P. H. Coding assignment and nucleotide sequence of simian rotavirus SA11 gene segment 10: location of glycosylation sites suggests that the signal peptide is not cleaved. J Virol. 1983 Nov;48(2):335–339. doi: 10.1128/jvi.48.2.335-339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J., Bobek L., Brennan V., Held W. Yeast viral RNA polymerase is a transcriptase. Nucleic Acids Res. 1980 Jul 11;8(13):2985–2997. doi: 10.1093/nar/8.13.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar L. W., Esparza J., Hudson G. R., Chmelo R., Lee P. W., Joklik W. K. Cloning the double-stranded RNA genes of reovirus: sequence of the cloned S2 gene. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7644–7648. doi: 10.1073/pnas.79.24.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. B., Pastan I., de Crombrugghe B. Sequence rearrangement and duplication of double stranded fibronectin cDNA probably occurring during cDNA synthesis by AMV reverse transcriptase and Escherichia coli DNA polymerase I. Nucleic Acids Res. 1980 Jul 11;8(13):3055–3064. doi: 10.1093/nar/8.13.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. J., Bobek L. A., Brennan V. E., Reilly J. D., Bruenn J. A. There are at least two yeast viral double-stranded RNAs of the same size: an explanation for viral exclusion. Cell. 1982 Nov;31(1):193–200. doi: 10.1016/0092-8674(82)90419-6. [DOI] [PubMed] [Google Scholar]
- Field L. J., Bruenn J. A., Chang T. H., Pinhasi O., Koltin Y. Two Ustilago maydis viral dsRNAs of different size code for the same product. Nucleic Acids Res. 1983 May 11;11(9):2765–2778. doi: 10.1093/nar/11.9.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Yeast virus-like particles possess a capsid-associated single-stranded RNA polymerase. Nature. 1977 Aug 4;268(5619):464–466. doi: 10.1038/268464a0. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Imai M., Richardson M. A., Ikegami N., Shatkin A. J., Furuichi Y. Molecular cloning of double-stranded RNA virus genomes. Proc Natl Acad Sci U S A. 1983 Jan;80(2):373–377. doi: 10.1073/pnas.80.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of DNA sequence analysis programs for microcomputers. Nucleic Acids Res. 1982 Jan 11;10(1):51–59. doi: 10.1093/nar/10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J., Hannig E. M., Leibowitz M. J. Multiple L double-stranded RNA species of Saccharomyces cerevisiae: evidence for separate encapsidation. Mol Cell Biol. 1984 Jan;4(1):92–100. doi: 10.1128/mcb.4.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J. D., Leibowitz M. J., Wickner R. B. Virion DNA-independent RNA polymerase from Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Jun 11;8(11):2349–2363. doi: 10.1093/nar/8.11.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]