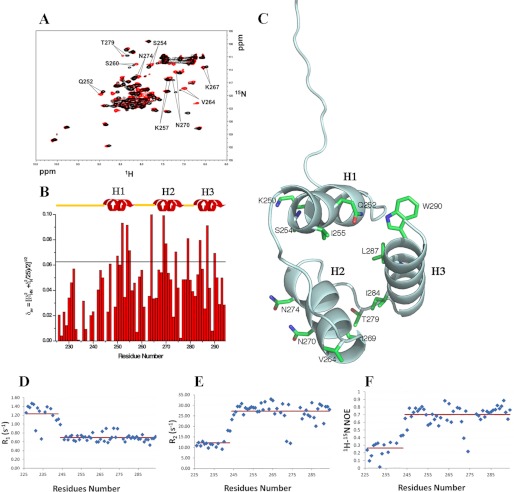
FIGURE 3.
Interaction of NPM1-C70 with the Pu24I G-quadruplex. A, 15N HSQC spectra of the protein before (black) and after addition of stoichiometric amounts of unlabeled Pu24I (red). Representative chemical shift variations are labeled, indicating relevant residues. B, chemical shift variations cluster in the three-helix bundle, whereas they are not found at the N-terminal 225–242 segment. The horizontal black line indicates the average chemical shift variation plus one standard deviation upon Pu24I addition. C, residues experiencing chemical shift variations higher than the average plus one standard deviation are highlighted on the structure of the protein. Residues belonging to helices H1 and H2 are solvent-exposed. A few hydrophobic residues belonging to helix H3 are also affected, indicating coupling between the helices upon Pu24I binding. Heteronuclear 15N-1H R1 (D) and heteronuclear 15N-1H R2 values (E) for NPM1-C70 in complex with Pu24I indicate that the N-terminal region flanking the three-helix bundle remains unstructured after Pu24I binding. F, the increase of heteronuclear 15N{1H} NOE values in the three-helix bundle upon Pu24I binding (see Fig. 1D for comparison) suggests increased rigidity.