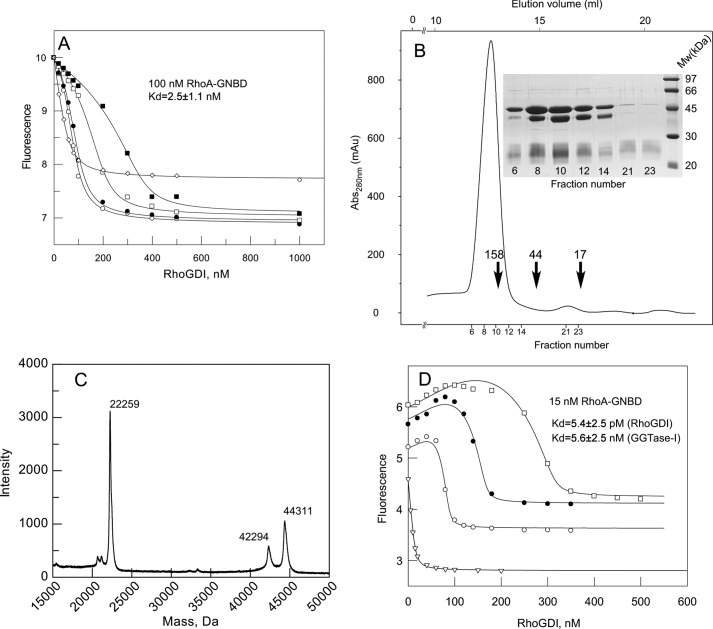
FIGURE 4.
Interaction analysis of prenylated RhoA·GDP with RhoGDI. A, titration of the RhoGDI into a mixture of fluorescently labeled 100 nm RhoA-GNBD (λex/em 479/560 nm) in the absence (open diamonds) and presence of increasing concentrations of RhoA-F: 50 (open circles), 100 (filled circles), 200 (open squares), and 400 nm (filled squares). The data were fitted to a competitive model, resulting in a Kd value of 2.5 nm. B, purification of the RhoA-GG·GGTase-I complex by size exclusion chromatography and SDS-PAGE analysis of complex containing fractions. Arrows indicate elution volumes of molecular weight standards. C, the MALDI-MS analysis of the purified RhoA-GG·GGTase-I complex. D, titration of RhoGDI to a 15 nm solution of RhoA-GNBD in the absence (open triangles) or presence of 100 (open circles), 200 (filled circles), or 400 nm (open squares) RhoA-GG·GGTase-I complex. The data were fitted globally by numerical simulation to a competitive binding model in the program Dynafit 4.0.