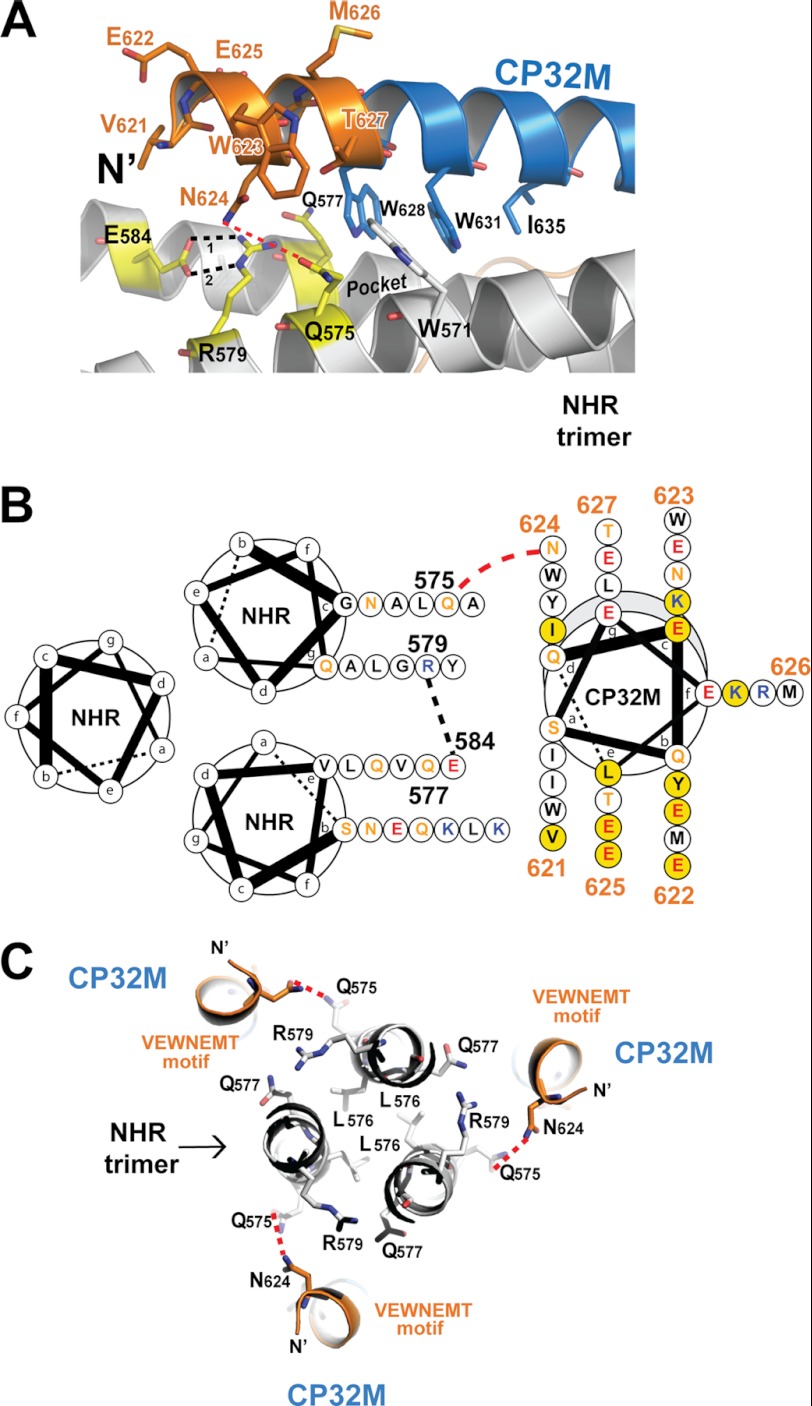
FIGURE 6.
The VEWNEMT motif of CP32M targets a hydrophilic region on the NHR trimer and form a novel layer of the 6-HB structure. A, a portion of a ribbon model of the 6-HB structure formed by the NHR546–588/CP32M chimera (positioned horizontally). The residues on the N-terminal VEWNEMT motif of CP32M, the residues of the pocket-binding domain, and the residues of the NHR binding site of CP32M are shown in stick model with labels. The hydrophilic ridge comprising Gln-575, Gln-577, Glu-584, and Arg-579 is colored in yellow. The hydrophobic pocket on NHR is indicated. Hydrogen bonds 1 and 2 between Glu-584 and Arg-579 are indicated by black dashed lines. The possible hydrogen bond between Asn-624 and Gln-575 is indicated by a red dashed line. B, helical wheel presentation showing the interaction between CP32M and the NHR trimer. Three NHR helices and one CP32M helix are shown as helical wheel projections. The view is from the bottom of the 6-HB. Three NHR helices form the central coiled coil, and a CP32M helix is packed against the interhelical groove between two NHR helices. At the top of the complex, the N terminus of CP32M (gray shading) is slightly tilted toward the upper NHR helix, and at the bottom of the complex, the C-terminal of the CP32M is tilted toward the lower NHR helix. The color code for the residues is: black, hydrophobic; orange, uncharged; blue, positively charged; red, negatively charged. Residues on the VEWNEMT motif of CP32M are labeled with the residue numbers. Residues mutated from the parental sequence to generate CP32M are highlighted by a yellow background. Red dashed lines indicate the possible hydrogen bonds between Asn-624 on CP32M and Gln-575 on the NHR helices. Black dashed lines indicate the hydrogen bond between Glu-584 and Arg-579. C, the VEWNEMT motif of CP32M forms the novel layer of the 6-HB. Three Leu-576 residues form the hydrophobic core of the NHR trimer that is locked by three Glu-584–Arg-579 “hasps” (the side chain of Glu-584 comes from the front layer and thus is not visible here; see A). Gln-575, Gln-577, Glu-584, and Arg-579 form a hydrophilic region on the NHR trimer, which mediates the hydrophilic interaction with Asn-624 on the VEWNEMT motif of CP32M. The possible hydrogen bonds between Asn-624 and Gln-575 are indicated by red dashed lines.