FIGURE 6.
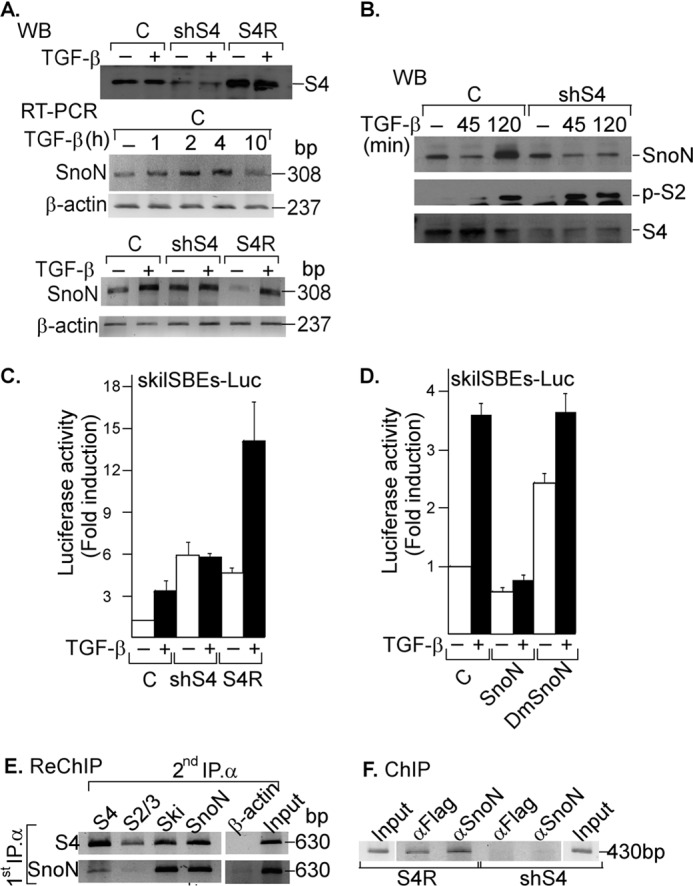
SNON depends on SMAD4 protein for SKIL promoter binding and repression. A, control AD293 cells (C) or cells stably expressing shSmad4 (shS4) or shSmad4 plus rescue S4 cDNA (S4R) were incubated for 2 h with or without 300 pm TGF-β. Whole cell protein extracts were subjected to IP with anti-S4 specific antibody followed by immunoblotting (upper panel). Total RNA was also isolated from control AD293 cells (upper and lower panels) or from shS4 or S4R AD293 cells (lower panel) incubated with or without 300 pm TGF-β for different times, and SNON (308-bp) and β-actin (237-bp) mRNAs were amplified by RT-PCR with specific primers (n = 2). B, control AD293 cells or stably expressing shSmad4 (shS4) AD293 cells were incubated for different times with 300 pm TGF-β. Whole cell protein extracts were subjected to IP with anti-SNON, anti-S2/S3, or anti-S4 antibody followed by WB with anti-SNON, anti-phospho-S2 (p-S2), or anti-S4 antibody (n = 2). Control or shS4 AD293 cells were transiently transfected with skilSBEs(408)-Luc reporter plasmid with or without plasmids bearing cDNA for SMAD4 rescue (S4R) (C), and AD293 cells were transiently transfected with skilSBEs(408)-Luc reporter along with plasmids bearing cDNA for WT SNON or DmSNON (D). Twenty-four hours post-transfection, cells were incubated for 12 h in the absence or presence of 100 pm TGF-β. Luciferase activity was normalized using β-gal expression and is reported as -fold induction over control. Values are mean ± S.E. (error bars) (n = 6). AD293 cells were transiently transfected with skilSBEs-Luc reporter, and 48 h post-transfection, a re-ChIP assay was carried out (n = 2) (E), or a ChIP assay was carried out in shS4 and S4R AD293 cells (F); both assays were performed using anti-S4, anti-FLAG, or anti-SNON antibody for the first IP; anti-S4, anti-S2/S3, anti-SNON, or anti-SKI antibody for the second IP; and anti-β-actin antibody as a control. PCRs were performed with primers for pGL3 plasmid bearing SKIL promoter (630 bp) or for SKIL promoter (430 bp).
