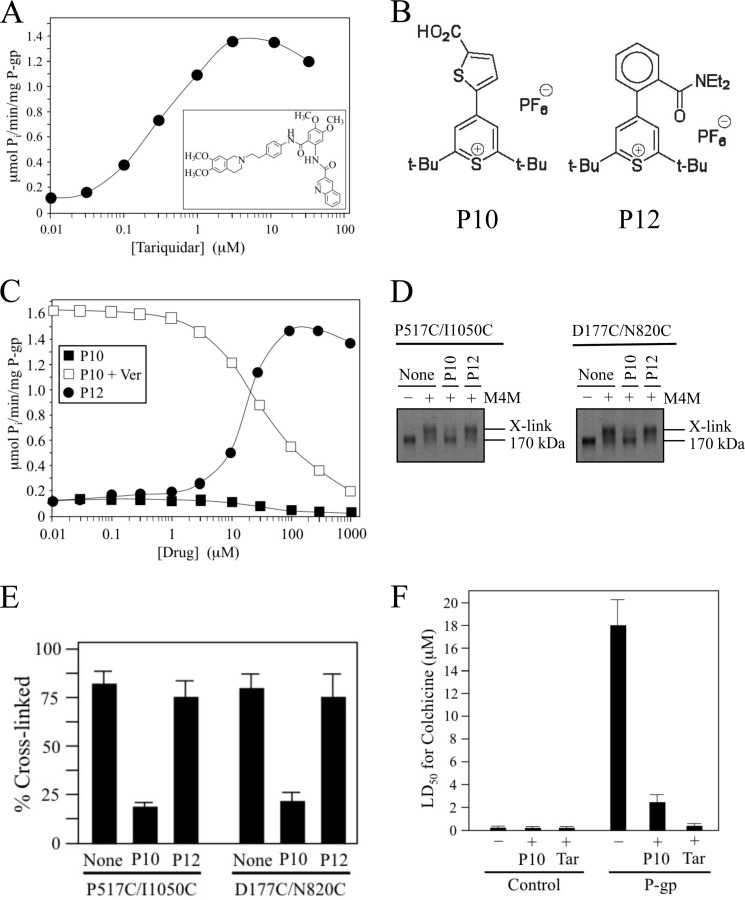
FIGURE 5.
Pyrylium compound P10 inhibits NBD cross-linking, ATPase activity and P-gp-mediated drug resistance. A, ATPase activity of isolated histidine-tagged Cys-less P-gp was determined in the presence of various concentrations of tariquidar (structure shown in inset). B, structures of pyrylium compounds P10 and P12. C, Cys-less P-gp ATPase activity was determined in the presence of various concentrations of P10 or P12. Inhibition of verapamil-stimulated ATPase activity was performed in the presence of 0.1 mm verapamil and increasing concentrations of P10. D, membranes prepared from HEK 293 cells expressing mutants P517C(NBD1)/I1050C(NBD2) or D177C(ICL1)/N820C(ICL3) were pretreated in the absence (None) or presence of 0.1 mm P10 or P12. The membranes were then treated with (+) or without (−) 0.05 mm M4M cross-linker and samples subjected to immunoblot analysis. The positions of cross-linked (X-link) and mature (170 kDa) forms of P-gp are indicated. E, percent of cross-linked product relative to total P-gp (cross-linked plus 170 kDa P-gp) was determined. Each value is the mean ± S.D. (n = 3). F, BHK cells (Control) or BHK cells expressing wild-type P-gp were incubated in the presence of various levels of colchicine in the presence (+) or absence (−) of 5 μm P10 or 100 nm tariquidar and the concentration of colchicine required to reduce cell viability by 50% (LD50) was determined. The results represent the average LD50 obtained from analysis of three independent assays ± S.D.