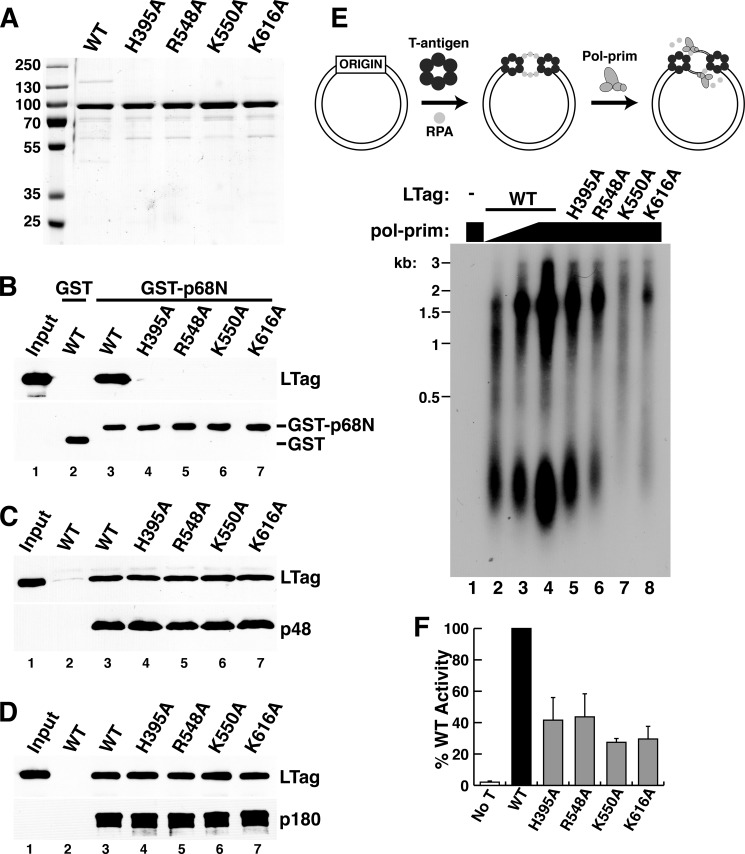
FIGURE 5.
Specific role of LTag-p68 interaction in primosome activity. A, purified full-length WT and the indicated mutant LTags were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. Marker proteins are shown at left. B, glutathione-agarose beads bound to either GST (lane 2) or GST-p68N (lanes 3–7) were incubated with full-length WT or the indicated point mutant LTags. Retained proteins were analyzed by Western blot with the indicated antibodies. Lane 1, 15% of the LTag input used for pulldowns in lanes 2–7. C and D, FLAG beads without (lane 2) or with bound p48/His6-FLAG×2-p58 heterodimer (C) or SJK237-31-Sepharose beads without (lane 2) or with bound p180 (D) were incubated with soluble, purified full-length WT or the point mutant LTags as indicated. Lane 1 shows 7.5% of the LTag input used for pulldowns in lanes 2–7. E, initiation of SV40 DNA replication initiation was assayed in monopolymerase reactions containing purified LTag, RPA, topoisomerase, and Pol-prim (diagram). Radiolabeled products of reactions lacking LTag (lane 1) or containing 300 ng of WT or the indicated mutant LTags (lanes 2–8) and varying amounts of Pol-prim (lane 2, 125 ng; lane 3, 250 ng; lanes 1 and 4–8, 500 ng) were analyzed by denaturing gel electrophoresis and phosphorimaging. DNA size markers are indicated (kb). F, initiation activity from three independent experiments as in E was quantified by phosphorimaging, and the activity of each mutant LTag was expressed relative to that of the WT LTag activity in each experiment. No T, as a negative control, LTag was omitted from the sample. Error bars indicate standard deviation.