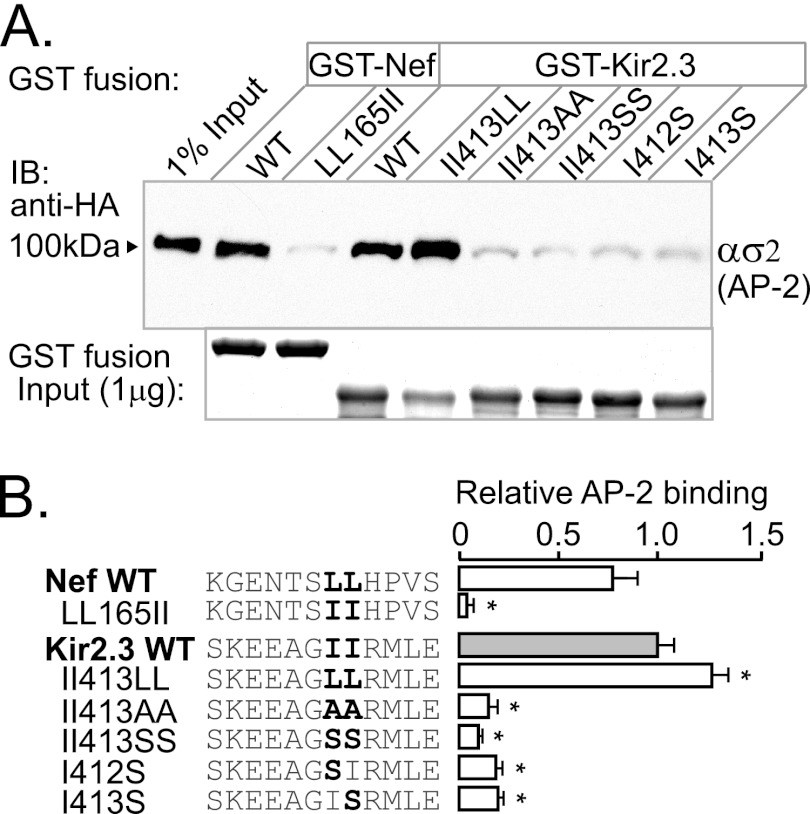
FIGURE 2.
Endocytic motif in Kir2.3 accepts Leu in place of Ile. To investigate the specific requirement for isoleucine in the Kir2.3 motif, the residues were replaced with leucine, alanine, or serine, and the mutant forms were tested for ασ2 interaction in GST pulldown assays. A, representative Western blots (upper panel) and Coomassie Blue staining of GST fusion proteins (lower panel). IB, immunoblot. B, densitometric quantification and summary of the data from three separate experiments (mean ± S.E.; *, p < 0.05). The II413LL mutation modestly increased binding to ασ2, whereas LL165II in Nef completely prevented binding. By contrast, the II413SS mutation decreased binding, similar to II413AA. Individual substitution of Kir2.3 Ile412 or Ile413 with serine blocked interaction, revealing that both Ile0 and Ile+1 are essential for binding.