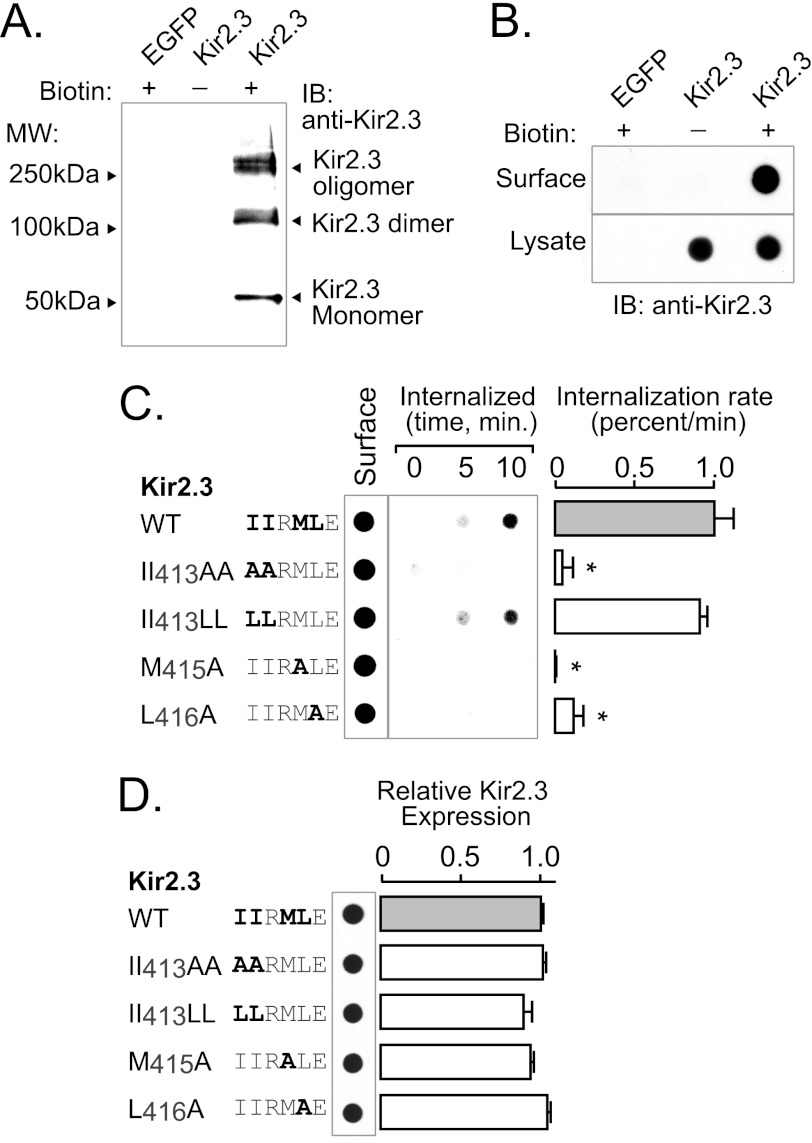
FIGURE 7.
TDH motif (IIXML416) is required for internalization of Kir2.3. Rates of endocytosis were determined in HEK293 cells expressing Kir2.3 (WT or mutant) using a surface biotin labeling/internalization chase assay. A, surface-biotinylated Kir2.3 was specifically detected in Western blots with anti-Kir2.3 antibodies. Shown is avidin-bound material from HEK293 cells transfected with Kir2.3 or enhanced GFP (EGFP) and surface-labeled with a cell-impermeant biotin derivative (+) or vehicle (−). Biotinylated Kir2.3 migrated on SDS-polyacrylamide gels as three species: a 50-kDa (monomeric) band, a 100-kDa (dimeric) band, and a higher molecular mass oligomeric form. Identical molecular mass forms were specifically detected in Western blots of whole cell lysates of Kir2.3-transfected HEK293 cells (data not shown). B, dot-immunoblot analysis of Kir2.3 with anti-Kir2.3 antibodies specifically detected the biotinylated channel at the cell surface. Avidin-purified material was compared with the whole lysate of cells transfected with Kir2.3 or enhanced GFP and surface-labeled with biotin (+) or vehicle (−). In contrast to Kir2.3-transfected cells, nothing was detected in cells transfected with Kir2.3 and labeled with vehicle or in enhanced GFP-transfected cells labeled with biotin, verifying specificity. C, a representative biotin internalization experiment is shown (left panel), next to the summary data (right panel). In these studies, surface channels were labeled with the cell-impermeant biotin derivative in the cold and then incubated at 37 °C for 0, 5, or 10 min to capture the initial rate of endocytosis. Biotin remaining at the cell surface was cleaved with MesNa, and internalized (biotinylated) Kir2.3 was recovered and detected along with the total surface pool in dot immunoblots. The internalization rate of mutant II413AA, M415A, and L416A channels was greatly reduced compared with WT and mutant II413LL Kir2.3 channels (mean ± S.E.; *, p < 0.05). IB, immunoblot. D, by contrast, mutation of the hydrophobic residues involved in AP-2 binding and endocytosis did not affect total Kir2.3 protein abundance.