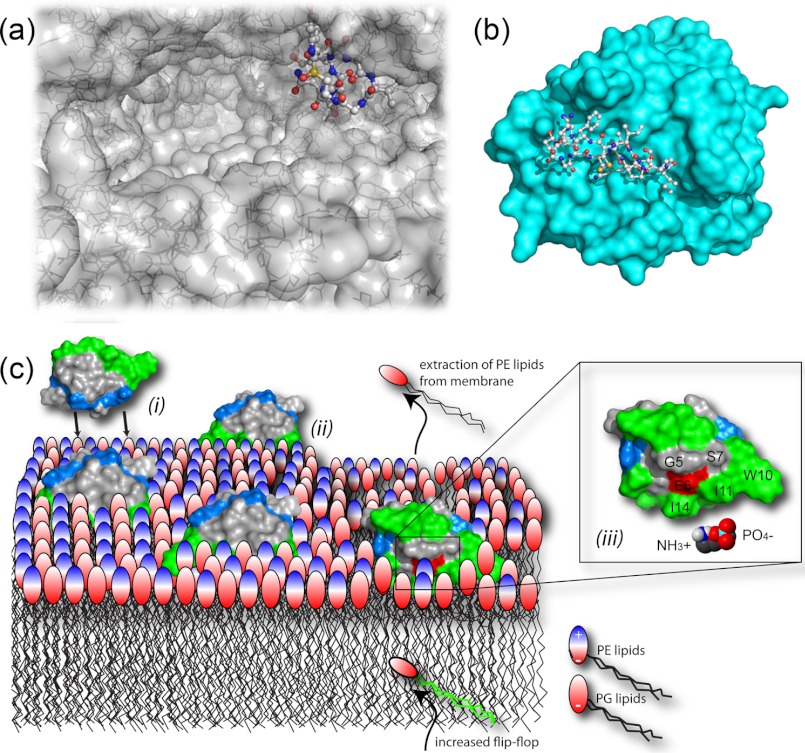
FIGURE 2.
Mechanisms of action. a, the crystal structure (Protein Data Bank code 3CQZ) shows α-amanitin binding deep in the substrate-binding channel of the large multisubunit RNA polymerase II. b, SFTI-I binds tightly to the trypsin active site (Protein Data Bank code 1SFI). A number of studies have demonstrated the ability of cyclotides to interact with and disrupt biological membranes. c, schematic model of the interaction between cycloviolacin O2 and E. coli inner membranes comprising phosphatidylglycerol (PG) and PE lipids. Accumulation of cyclotides, driven by electrostatic interaction ((i)), leads to membrane thinning ((ii)) through selective binding to and extraction of PE lipids ((iii)) and increased flip-flop of PE lipids from the inner leaflet.