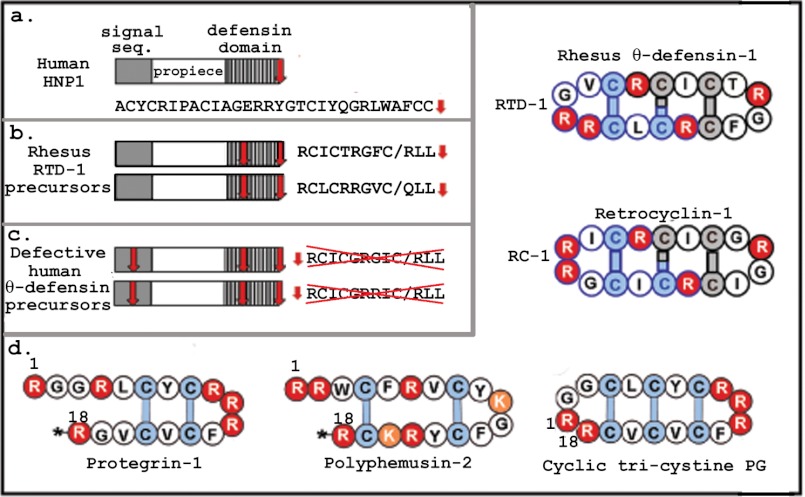
FIGURE 1.
Panel a shows the layout of the prepropeptide of a human α-defensin, HNP-1, as well as the sequence (seq.) of its C-terminal defensin domain. The red arrow represents a stop codon. Panel b shows the corresponding layout and sequences for RTD-1. An additional stop codon (red arrow) limits the initial defensin domain peptides to 12 residues, three of which will be removed during subsequent post-translational processing. The mature cyclic RTD-1 peptide is composed of two different nine-residue precursors. Panel c shows the human counterparts of RTD-1. The transcripts contain an additional stop codon in the signal sequence domain. This mutation aborts translation of the θ-defensin peptide precursors. Panel d shows simplified structural diagrams of five structurally related antimicrobial peptides. In these diagrams, arginines are red, lysines are orange, and cysteines are blue. Asterisks signify amidated C-terminal arginines. Polyphemusin-2 was isolated from the white blood cells of the horseshoe crab Limulus polyphemus. It has impressive antimicrobial properties and is active against HIV-1 (50, 51). The other four peptides are discussed in text.