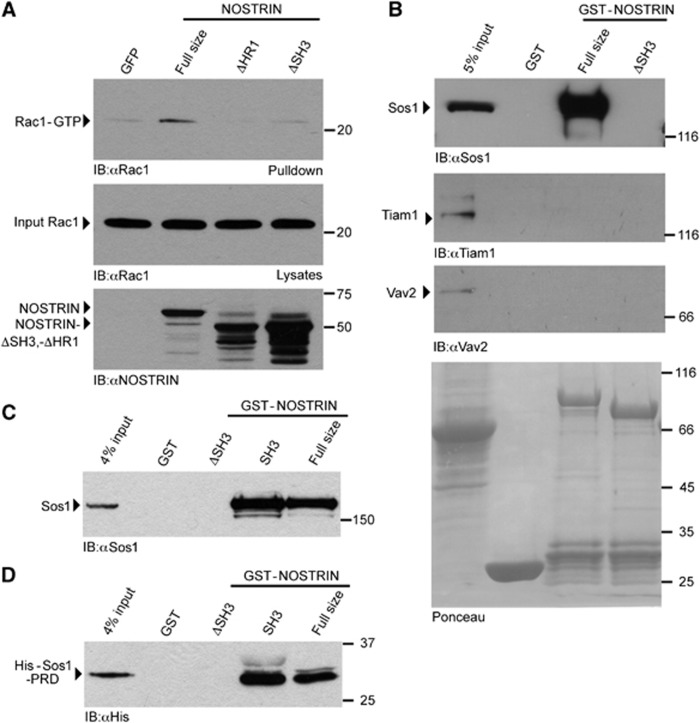
Figure 5.
NOSTRIN interacts with the Rac1 GEF Sos1 and induces Rac1 activation. (A) NOSTRIN overexpression induces Rac1 activation depending on the presence of the HR1 and the SH3 motif. NOSTRIN, NOSTRINΔHR1, NOSTRINΔSH3 or GFP were expressed using the SFV-system and the activity of Rac1 measured as amount of Rac1-GTP precipitated with the CRIB domain of PAK (PAK-CRIB assay). Equal amounts of Rac1 (input Rac1) and NOSTRIN, NOSTRINΔHR1 and NOSTRINΔSH3 were applied. Overexpression of NOSTRIN induced strong Rac1 activation in comparison to GFP, the deletion mutants NOSTRINΔSH3 and NOSTRINΔHR1 did not induce Rac1 activation. (B) NOSTRIN interacts with Sos1. GST-pulldown from primary mouse lung endothelial cells using GST-NOSTRIN, GST-NOSTRINΔSH3 or GST alone indicated specific interaction of full size NOSTRIN with endogenous Sos1, but not with Tiam1 or Vav2. Deletion of the SH3 domain in NOSTRINΔSH3 resulted in loss of the NOSTRIN/Sos1 interaction. (C) NOSTRIN SH3 domain is sufficient for interaction with Sos1. GST-pulldown from cell lysate using GST-NOSTRIN, GST-NOSTRINΔSH3, GST-SH3 or GST alone indicated specific interaction of endogenous Sos1 with GST-NOSTRIN and the isolated SH3 domain GST-SH3. (D) NOSTRIN SH3 domain binds the proline-rich domain of Sos1. GST-pulldown with recombinantly expressed and purified proline-rich domain of Sos1 (His-Sos1-PRD) confirmed specific and direct interaction of Sos1 with GST-NOSTRIN and GST-SH3.