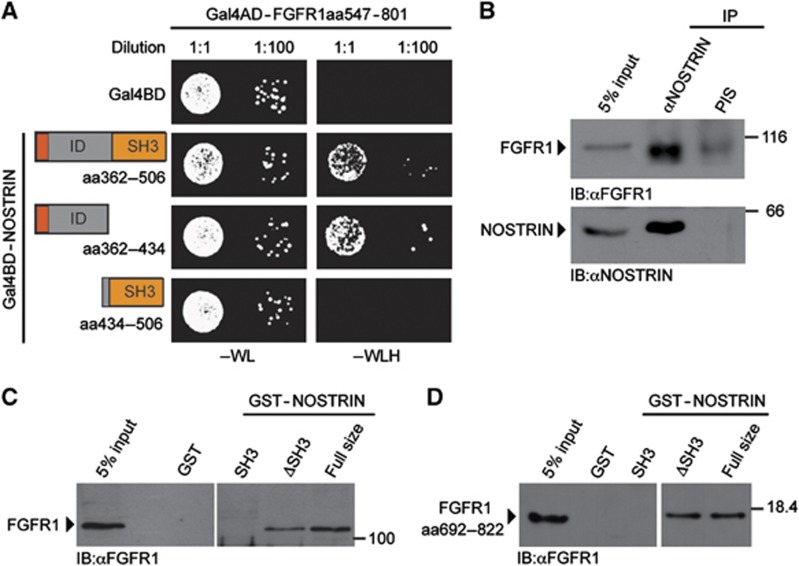
Figure 6.
NOSTRIN interacts with FGFR1 (A) Y2H interaction analysis between FGFR1 and NOSTRIN. Co-expression of a fusion protein of the Gal4 activator domain (Gal4AD) with the cytoplasmic tail of the FGFR1 (Gal4AD-FGFR1aa547–801) with 3 separate fusion proteins between the Gal4 DNA binding domain (Gal4BD) with distinct C-terminal NOSTRIN fragments (Gal4BD-NOSTRINaa362–506, Gal4BD-NOSTRINaa362–434 and Gal4BD-NOSTRINaa434–506). Gal4BD co-transformed with Gal4AD-FGFR1aa547–801 served as control. Each co-transformed yeast clone was spotted onto growth medium devoid of tryptophan and leucin (−WL) and growth medium devoid of tryptophan, leucine and histidine (−WLH) in 2 different dilutions. Growth of co-transformed yeast colonies on −WL indicates lack of toxicity, growth on −WLH of yeast co-transformants with Gal4AD-FGFR1aa547–801 in combination with either Gal4BD-NOSTRINaa362–506 or Gal4BD-NOSTRINaa362–434 indicates interaction, while no growth of yeast co-transformants with Gal4AD-FGFR1aa547–801 in combination with Gal4BD-NOSTRINaa434–506 indicates a lack of interaction. (B) NOSTRIN interacts with FGFR1 in mammalian cells. Co-immunoprecipitation of endogenous FGFR1 with endogenous NOSTRIN from cell lysates using a polyclonal NOSTRIN-specific antiserum for immunoprecipitation (IP). Lack of co-immunoprecipitation with pre-immune serum (PIS) served as specificity control. 5% of the volume of the cell lysate used for IP is shown for comparison of protein levels (5% input). Proteins are detected by immunoblotting with FGFR1-specific antiserum and a NOSTRIN-specific antibody (Mookerjee et al, 2007). (C) NOSTRIN interacts with FGFR1 independently of the SH3 domain. GST-pulldown experiment using GST-NOSTRIN, GST-NOSTRINΔSH3, GST-SH3 or GST alone to pulldown endogenous FGFR1 from cell lysates. Lack of interaction with GST indicates specificity. Proteins are detected by immunobloting with polyclonal FGFR1-specific antiserum. (D) Direct protein/protein interaction analysis. Recombinantly expressed and purified GST-NOSTRIN full size, GST-NOSTRINΔSH3, GST-SH3 or GST used in combination with a recombinantly expressed and purified C-terminal fragment of FGFR1 comprising aa 692–822, confirming interaction of FGFR1 with GST-NOSTRIN and GST-NOSTRINΔSH3 (FGFR1 aa692–822 was chosen because it could be purified as a soluble protein in sufficient amounts). Proteins were detected by immunoblotting with an FGFR1-specific antibody.