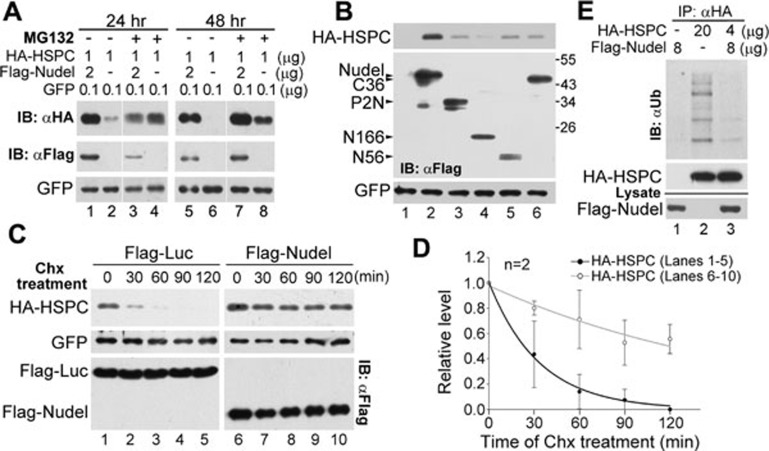
Figure 3.
Nudel stabilizes HSPC300. (A) Nudel protected HSPC300 from the proteasome degradation. HEK293T cells were transfected with the indicated amount of plasmids to overexpress HA-HSPC300 and GFP with or without Flag-Nudel. The cells were harvested at the indicated time after 12 h of the MG132 treatment (5 μM), followed by immunoblotting (IB). GFP served as a control for the transfection efficiency. (B) Nudel mutants failed to stabilize HSPC300. HEK293T cells were transfected as indicated in A to transiently coexpress Flag-tagged Nudel or its mutants with HA-HSPC300. Cells were collected at 48 h post transfection for immunoblotting. A diagram for the Nudel mutants is shown in Supplementary information, Figure S2A. The effect of NudelP2C, which interacted with HSPC300 (Supplementary information, Figure S2A and S2C), was not examined due to its extremely low expression levels. (C) Nudel attenuated the turnover rate of HSPC300. HEK293T cells were transfected with the plasmids as indicated in A, except that Flag-luciferase was expressed in the control cells. The cells were treated with 200 μg/ml of Chx at 24 h post transfection for the indicated times, followed by immunoblotting. (D) The quantification results for the relative protein levels of HA-HSPC300 in C. Protein levels were normalized to that of GFP and presented relative to the values at 0 min. (E) Nudel attenuated the polyubiquitination of HSPC300. HEK293T cells transfected for 36 h to overexpress the indicated proteins were treated with MG132 for an additional 12 h. The cells were lysed in the RIPA buffer and then subjected to IP using the anti-HA resin.