Table 1.
Characteristics of controlled feeding trials investigating the effect of ‘catalytic’ doses (≤36 g/d) of fructose on cardiometabolic endpoints
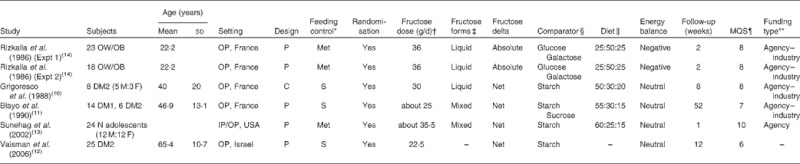
MQS, Methodological Quality Score; OW/OB, overweight/obese; OP, outpatient; P, parallel; Met, metabolic; DM2, type 2 diabetes mellitus; M, male; F, female; C, cross-over; S, supplement; DM1, type 1 diabetes mellitus; N, normal; IP, inpatient.
Met feeding control represents the provision of all meals, snacks and study supplements (test sugars and foods) during the study. S feeding control represents the provision of study supplements.
Doses preceded by ‘about’ represent average doses, where fructose was administered on a % energy or g/kg body-weight basis.
Fructose was provided in one of two forms: (1) a liquid form, where all or most of the fructose was provided as beverages or crystalline fructose to be added to beverages, or (2) in a mixed form, where all or most of the fructose was provided as beverages, solid foods and/or crystalline fructose to be added to beverages and/or foods.
Comparator refers to the reference carbohydrate (glucose, galactose, starch or sucrose).
Values are for the ratio of carbohydrate:fat:protein.
The Heyland MQS assigns scores from 0 to 1 or 0 to 2 over nine categories of quality related to study design, sampling procedures and interventions for a total of thirteen points. Trials scored ≥ 8 were considered high quality(8).
Agency funding represents funding from government, university or not-for-profit health agency sources. None of the trialists declared any conflicts of interest.
