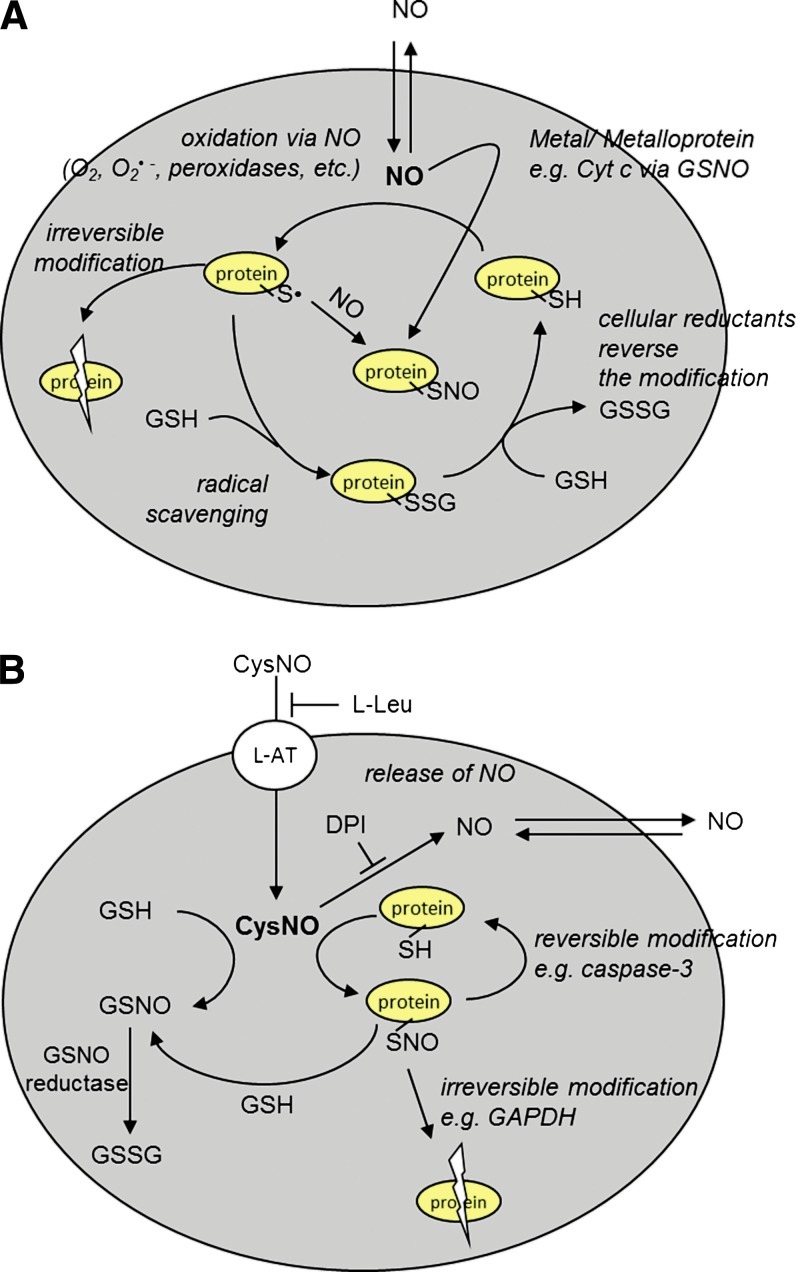
FIG. 5.
Model for NO and S-nitrosothiol-dependent cellular effects. (A) NO can oxidatively modify intracellular thiols (e.g., by forming thiyl radical) after reacting with O2, O2•−, or through its interaction with peroxidases. Cellular-reducing machinery reacts with the free radical, thus prohibiting further damage to the protein (e.g., glutathionation). Glutathionation is a transient modification that can be reduced back to the parent thiol. When protein thiyl radical is formed, it can react with NO to form S-nitrosothiol or lead to irreversible protein damage due to the oxidation of adjacent amino acids. S-nitrosothiol may also be formed by a more concerted mechanism involving NO, a metal center, and a thiol. (B) CysNO is transported into the cell through L-AT in an L-leucine-inhibitable process. Inside the cell, CysNO can undergo transnitrosation with protein thiols and GSH. The formation of protein-associated S-nitrosothiols can be either reversible (e.g., caspase-3) or facilitate further reactions, leading to the irreversible inhibition of a given protein (e.g., GAPDH). CysNO can release NO in a process that can be inhibited by DPI, an inhibitor of flavin-containing enzymes. GSNO, formed from GSH on transnitrosation with CysNO or protein S-nitrosothiol, is metabolized by GSNO reductase. DPI, diphenyleneiodonium chloride; GAPDH, glyceraldehyde-3 phosphate dehydrogenase. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars).