Abstract
S-nitrosylation is a redox-sensitive protein modification, which is a highly specific, but reversible mechanism that regulates several signal transduction cascades. Oxidative stress plays a causal role in the ototoxic effects of an anti-neoplastic drug, cisplatin. Despite emerging evidence implicating nitroxidative stress as a critical factor in cisplatin toxicity, the significance of the cochlear protein S-nitrosylation in cisplatin ototoxicity is yet to be understood. In the present study, a 16-mg/kg dose of cisplatin, induced a significant shift in the amplitudes of distortion product otoacoustic emissions, a measure of outer hair cell activity, in Wistar rats, 3 days post-treatment. These ototoxic effects were accompanied by significant increases in the S-nitrosylation of at least three cochlear proteins. Biological significance of these S-nitrosylated proteins was indicated by their immunolocalization in organ of Corti, stria vascularis, and spiral ganglions, which are known cochlear targets of cisplatin toxicity. In addition, co-treatment with Trolox, an inhibitor of peroxynitrite, attenuated cisplatin-induced S-nitrosylation of cochlear proteins and prevented the associated hearing loss. The cisplatin-induced S-nitrosylation of inner ear proteins, their sensitive cochlear localization, and their potential association with cisplatin-induced hearing loss suggests that S-nitrosylation of cochlear proteins might play a crucial role in mediating cisplatin ototoxicity. Antioxid. Redox Signal. 17, 929–933.
Introduction
S-nitrosylation is a post-translational modification of significant physiological as well as pathological implications as it regulates protein function (3). It is an important sequel of cellular nitrosative stress and is highly specific with precisely regulated and targeted downstream effects. S-nitrosylation occurs by covalent attachment of a nitric oxide (NO) group to cysteine residue of specific proteins (8). Unlike nitration, where a nitro (NO2) group binds irreversibly to tyrosine residue, S-nitrosylation is reversible, which allows it to serve as an on/off switch to precisely modify protein function in response to cellular signals. Denitrosylation occurs through enzyme-mediated reactions or nonenzymatically by changes in the redox environment of the protein. Depending on the levels of cellular oxidative stress, this reversible S-nitrosylation, which plays a crucial role in NO cell signaling, can progress to an irreversible sulphonic acid modification resulting in cellular toxicity (5).
Recent studies have reported nitroxidative modification of several inner ear proteins. Nitration of cochlear proteins has been reported in various ototoxic conditions associated with oxidative stress such as noise-induced, age-related, and drug-induced hearing loss. Cisplatin is one among the clinically useful drugs whose ototoxic side effects limit its therapeutic efficacy. This anti-neoplastic drug induces nitration of cochlear Lmo4, a potential biomarker of cisplatin-induced oxidative damage of the inner ear (7). Cisplatin-induced nitrosylation of Bcl2, a proto-oncogene and p53, a transcriptional factor that regulates apoptosis, has been characterized in nonauditory cells (2, 4). However, cisplatin-induced S-nitrosylation of cochlear proteins and their functional implications has not been examined so far. Since cisplatin damages the inner ear by forming DNA adducts, the identification of several DNA repair proteins as specific targets of S-nitrosylation is of great relevance to this study. Moreover, the emergence of cochlear nitroxidative stress as a crucial factor responsible for the ototoxic effects of cisplatin (7) suggests that S-nitrosylation of cochlear proteins is likely to have an important functional role in mediating cisplatin toxicity.
Innovation.
Protein S-nitrosylation is emerging as an important post-translational modification that plays a comparable role to that of phosphorylation, in several signal transduction cascades and in the regulation of cellular function (8). Pertinent to this study, S-nitrosylation is a central regulator of stress-induced apoptosis as it can signal either a pro- or an anti-apoptotic response, based on the characteristics of its substrate protein. Since the cytotoxic effects of cisplatin occur primarily through apoptosis, protein S-nitrosylation, which is implicated in both mitochondrial as well as nuclear programs of apoptosis (1), is poised to play an important role in cisplatin-induced hearing loss. The present study provides the first evidence of S-nitrosylation of cochlear proteins in cisplatin ototoxicity. Cisplatin treatment induced a significant increase in the S-nitrosylation of at least three different proteins in the cochlea. These S-nitrosylated proteins were immunolocalized in sensitive cochlear targets of cisplatin toxicity. Moreover, the cisplatin-induced S-nitrosylation of cochlear proteins was attenuated by Trolox co-treatment, which also prevented the hearing impairment. Collectively, these findings highlight the importance of nitrosative stress and indicate the significance of cochlear protein S-nitrosylation in cisplatin-mediated ototoxicity.
Cisplatin-Induced Ototoxicity
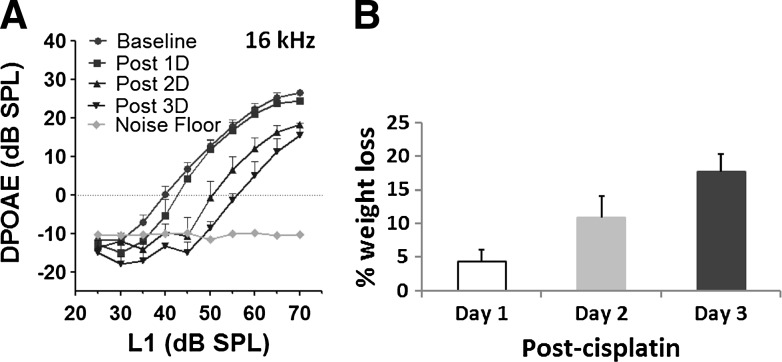
The ototoxic side effects of cisplatin have been well documented (6, 7, 9). Consistent with previous reports, a 16 mg/kg dose of cisplatin induced a significant shift in distortion product amplitudes (Fig. 1A), of about 10–15 dB, with a 16-kHz f2 primary tone, 3 days post-treatment. The gradual decrease in distortion product otoacoustic emissions (DPOAEs), observed 1, 2, and 3 days after cisplatin treatment suggested a cisplatin-induced time-dependent increase in hearing impairment due to the loss of outer hair cell (OHC) activity. Moreover, the time-dependent increase in weight loss observed 1, 2, and 3 days after cisplatin treatment (Fig. 1B) pointed to the toxic side effects of cisplatin. Reactive oxygen species have been shown to be crucial factors responsible for these ototoxic effects of cisplatin (9). Since, S-nitrosylation is a redox-based regulator of protein function and a principal effector of apoptosis (1), it is a potential mediator of cisplatin ototoxicity. To test this hypothesis, it is essential to determine cisplatin-induced S-nitrosylation of cochlear proteins.
FIG. 1.
Cisplatin-induced toxicity. (A) distortion product otoacoustic emissions (DPOAEs) recorded before and 1, 2, or 3 days after cisplatin treatment indicated a definite shift in DP amplitudes that gradually decreased with time. This indicates that cisplatin induces a time-dependent increase in hearing impairment. The results are expressed as mean±standard error, n=6. (B) Cisplatin also induced a time-dependent loss of body weight with the animals losing 15%–20% weight by the third day. The results are expressed as mean±standard deviation, n=6.
S-nitrosylation of Cochlear Proteins in Cisplatin Ototoxicity
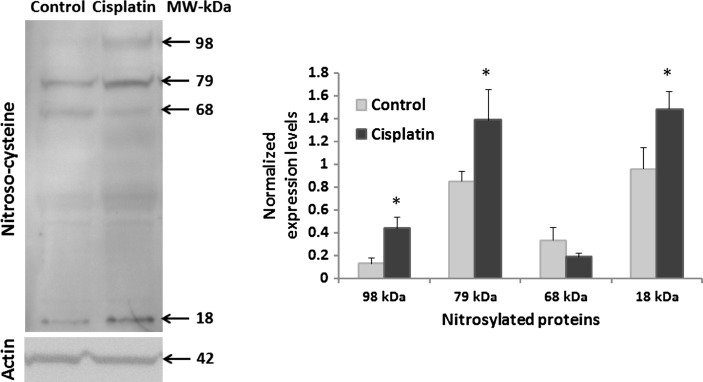
Increasing evidence indicates the functional significance of S-nitrosylation in regulating molecular signaling that determines cellular response to stress (3, 5). In the inner ear, S-nitrosylation of Ca2+ channel proteins have been reported to inhibit the Ca2+ currents and modulate the responses of vestibular hair cells. However, the functional significance of protein S-nitrosylation in the cochlea is poorly understood. To the best of our knowledge, the present study provides the first evidence of S-nitrosylation of cochlear proteins, as immunoblots with anti-S-nitroso-cysteine detected at least four different proteins in the cochlea. Of these, the cochlear expression of three S-nitrosylated proteins, at 98, 79, and 18 kDa apparent molecular weights, increased significantly (p<0.05), 3 days after cisplatin treatment (Fig. 2). The expression of a fourth S-nitrosylated protein, detected at an apparent molecular weight of 68 kDa, showed a tendency to decrease. The darker intensity of the proteins detected at 79 and 18 kDa probably indicates their relatively higher expression levels in cochlea although, it can be also attributed to a better immunoreactivity of the antibody to these specific proteins. Nevertheless, the immunoblots suggest that cisplatin induces an overall increase in S-nitrosylation of cochlear proteins. Cochlear expression of actin was used to normalize the expression levels of S-nitrosylated cochlear proteins.
FIG. 2.
Cisplatin-induced S-nitrosylation of cochlear proteins. Western blot analysis with anti-S-nitrosocysteine, 3 days after a 16 mg/kg cisplatin treatment, demonstrated S-nitrosylation of cochlear proteins in Wistar rats. Cisplatin induced significant increases in S-nitrosylation of at least three different cochlear proteins (*p<0.05). The specificity of the antibody detection was verified by the absence of S-nitrosylated protein bands when the membrane was treated with mercuric chloride before immunoblotting (not shown as a similar data is represented in Fig. 3). The immunoblots were normalized with the expression of actin. The numbers indicate the apparent molecular weights. The images are representative samples of three biological replicates and results are expressed as mean±standard error, n=3.
Cisplatin has previously been reported to nitrosylate proteins like Bcl2 and p53 in lung epithelial and colon carcinoma cells (2, 4). However, the apparent molecular weights of the S-nitrosylated cochlear proteins (98, 79, and 18 kDa) suggest that these modified cochlear proteins could be novel targets of cisplatin-induced S-nitrosylation. As accumulating evidence highlights the regulatory role of protein S-nitrosylation in determining the cellular responses (5, 8), these three cochlear proteins are likely to play an important role in mediating cisplatin ototoxicity. S-nitrosylation of apoptotic, pro-survival, and DNA repair proteins have been reported to alter the biological activity of the target proteins with vital functional implications. Cisplatin also modulates the cochlear expression of several apoptotic, survival, and DNA repair proteins (6). However, the nitroxidative modifications of these cochlear proteins have not been examined. It is possible that, putative S-nitrosylation of some of these target cochlear proteins or their upstream molecules modulates their cochlear expression and/or function to facilitate cisplatin-induced ototoxicity. Nonetheless, the localization of these S-nitrosylated proteins in the cochlea is critical to comprehend their functional implications.
Localization of S-nitrosylated Proteins in Sensitive Cochlear Regions
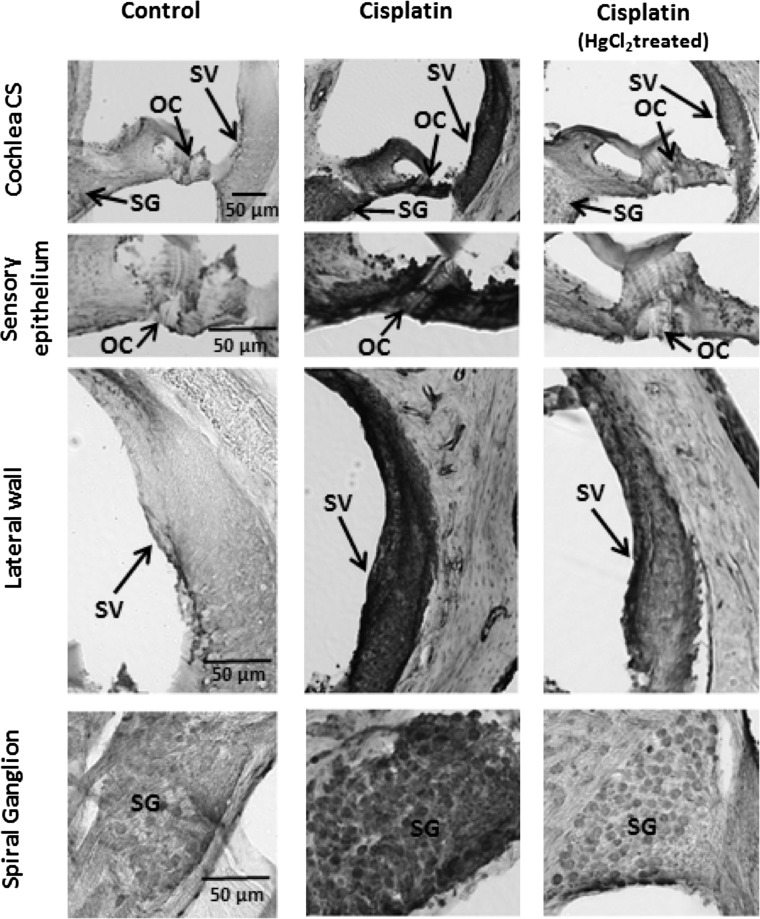
Cisplatin-induced increase in the expression of S-nitrosylated proteins was detected in organ of Corti, the sensory component; stria vascularis, the vascular component; and spiral ganglions, the neuronal component of the cochlea. The S-nitrosylated proteins were immunolocalized in the inner ear with anti-S-nitroso-cysteine (Fig. 3) by 3′3′diaminobenzidine tetrahydrochloride (DAB) immunostaining. Consistent with the immunoblots, the intensity of the immunostaing was markedly increased in the cisplatin-treated samples, 3 days post-treatment, suggesting a cisplatin-induced increase in the S-nitrosylation of cochlear proteins. The susceptibility of these cochlear regions to cisplatin ototoxicity has been well documented (9). Particularly, the organ of Corti consists of the key sensory receptor cells, the OHC, which is susceptible to cisplatin-induced functional as well as morphological loss, as demonstrated by DPOAEs and cochleograms (7). The localization of S-nitrosylated proteins in the cells of these highly sensitive cochlear regions that are known targets of cisplatin ototoxicity supports the significance of S-nitrosylation in cisplatin-mediated ototoxicity. Nevertheless, the potential impact of cisplatin-induced cochlear protein S-nitrosylation on associated hearing impairment would further highlight its clinical significance.
FIG. 3.
Immunolocalization of S-nitrosylated cochlear proteins. Nitrosylated cochlear proteins were immunolocalized in cryosectioned cochlea with anti-S-nitrosocysteine. Cisplatin-induced increase in nitrosylated proteins were localized using 3′3′diaminobenzidine tetrahydrochloride (DAB) staining in the cells of the organ of Corti (OC), stria vascularis (SV), and spiral ganglions (SG). The cisplatin-induced increase in immunostaining was diminished in adjacent sections that were pretreated with mercuric chloride. This indicates the specificity of the staining as Hgcl2 cleaves the S-NO linkage. The images are representative samples of three biological replicates.
Attenuation of Cochlear Protein S-nitrosylation and Prevention of Cisplatin-Induced Hearing Loss
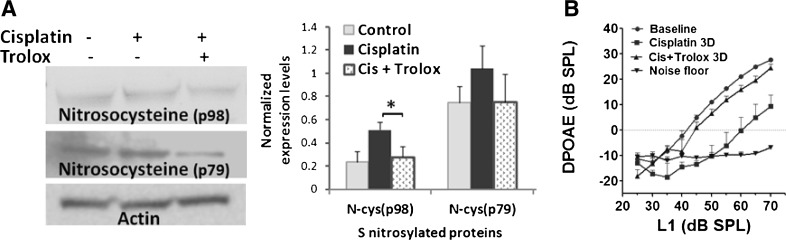
The functional importance of protein S-nitrosylation in cisplatin ototoxicity was indicated by the pharmacological modulation of cochlear protein S-nitrosylation with Trolox, an inhibitor of peroxynitrite (Fig. 4A). Treatment with 100 mg/kg/day dose of Trolox, which is an otoprotective agent widely employed in biological and biochemical applications to prevent oxidative stress and apoptosis, attenuated the cisplatin-induced S-nitrosylation of p98 (p<0.05) and showed a tendency to reverse the S-nitrosylation of p79 to control levels. Trolox treatment did not modulate the cisplatin-induced changes in p18 suggesting that the S-nitrosylation of this cochlear protein probably occurs through alternative mechanisms such as transnitrosylation. Cochlear expression of these proteins was normalized with that of actin. In addition to attenuating nitroxidative modification of proteins in the cochlea, Trolox, co-treatment, significantly reversed the cisplatin-induced shift in DPOAE amplitudes (p<0.05, Fig. 4B). The attenuation of cisplatin-induced S-nitrosylation of cochlear proteins and prevention of associated hearing loss, after co-treatment with Trolox, indicates the potential significance of S-nitrosylation in cisplatin ototoxicity. However, identification of the S-nitrosylated cochlear proteins is necessary to reveal the specific molecular targets and signaling mechanism by which cisplatin-induced S-nitrosylation of cochlear proteins mediate ototoxicity.
FIG. 4.
Attenuation of cisplatin-induced S-nitrosylation of cochlear proteins and prevention of hearing loss. (A) Trolox treatment attenuated the cisplatin-induced S-nitrosylation of p98 (*p<0.05) and showed a tendency to revert the changes in p79. Immunoblots are representative samples from 3 biological replicates and results are expressed as mean±standard error, n=3. Actin was used for normalization. (B) Prevention of cisplatin-induced hearing loss by Trolox is indicated by a reversal of cisplatin-induced shift in DPOAE amplitudes (p<0.05). The results are expressed as mean±standard error, n=4.
Conclusions and Future Directions
In summary, ototoxicity is an important side effect of the anti-cancer drug cisplatin. The critical role of cochlear nitrosative stress in cisplatin ototoxicity is indicated by cisplatin-induced S-nitrosylation of cochlear proteins that accompanies the ototoxic effects of cisplatin. Immunolocalization of S-nitrosylated proteins in the cochlear targets of cisplatin signifies the importance of protein S-nitrosylation in cisplatin ototoxicity. Furthermore, the attenuation of cochlear protein S-nitrosylation and prevention of cisplatin-induced hearing loss by Trolox co-treatment suggests the functional significance of protein S-nitrosylation in mediating cisplatin ototoxicity. Identification of the S-nitrosylated cochlear proteins will eventually clarify the specific protein-signaling pathway by which S-nitrosylation modulates cell death or cell survival responses in cisplatin ototoxicity.
Notes
Materials and methods
Animals
The Institutional Animal Care and Use Committee approved the experimental protocol, which followed the guidelines suggested by the National Institutes of Health. Three-month-old male Wistar rats (250–350 gm) from Charles River Laboratories (Wilmington, MA) were used for all the experiments. The animals were housed in the University at Buffalo—Laboratory Animal Facility with free access to food and water in a temperature-controlled room with 12 h light/dark cycle.
Reagents
All reagents were purchased from the Sigma Aldrich Chemical Company (St. Louis, MO) unless noted otherwise.
Drug treatment
Animals were treated with a single, 16 mg/kg dose of cisplatin by a slow intraperitoneal injection under anesthesia (isoflurane—4% induction, 1.5% maintenance, with 1 L/min O2). Cisplatin was mixed with 0.9% saline at 1 mg/ml concentration and injected at the rate of 10 ml/h using a syringe pump. The animals were weighed and hydrated daily with 15 ml 0.9% saline/kg. Trolox (100 mg/kg/d) was mixed with sterile saline (pH 7.2–7.4) and administered by the intra-peritoneal injection 1 h before and on the 1st and 2nd day after cisplatin treatment. All animals were sacrificed on the third day after cisplatin treatment (7).
Distortion product acoustic emission
Animals were anesthetized with isoflurane (4% induction, 1.5% maintenance with 1 L/min O2) and DPOAEs were measured at the cubic difference frequency 2f1-f2 using an Etymotics 10B plus probe (Etymotic Research, Inc., Elk Grove Village, IL) microphone and hardware and software from Intelligent Hearing Systems (IHS, Miami, FL). Stimuli was presented, holding L2=L1−10 dB and f2/f1=1.2, for L1 levels from 70 to 25 dB SPL in 5 dB increments. DPOAE was measured for f2 frequencies between 2 and 32 kHz, for each animal before and after cisplatin treatment (6, 7).
Cochlear protein extraction
Cochlea was taken out of the bulla after decapitating the animals anaesthetized with CO2 inhalation. Cochlear tissue was dissected out on ice-cold phosphate buffered saline (PBS) and cochlear proteins were extracted by homogenizing the cochlear tissue in the radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors and 5 mM EDTA (Pierce Chemical Co., Thermo Fisher Scientific, Rockford, IL). Total protein concentration was determined by the Bradford assay (6, 7).
Western blot
Cochlear proteins (20 μg) were denatured, separated on a 4%–12% gradient NuPage gels (Invitrogen, Carlsbad, CA) by electrophoresis and transferred to a polyvinylidene difluoride membrane using an iBlot Dry Blotting System (Invitrogen). The membranes were blocked and incubated with anti-S-Nitroso-Cysteine (#N5411, Sigma Aldrich, 1:500) for 1 h followed by incubation with a secondary antibody for 1 h. The protein bands were detected with chemiluminescent reagents (Pierce Chemical Co., Thermo Fisher Scientific) using a Fuji model LAS 1000 imaging system (Stamford, CT). The immunoblots were then stripped and reprobed with a monoclonal antibody against actin (Millipore, Bellerica, MA) to facilitate normalization. The blots were treated with 0.2% HgCl2 for 30 min at room temperature to test the specificity of antibody binding to S-nitrosylated proteins. One-tailed t-tests were employed to analyze the statistical significance between the control and treated groups (6, 7).
Immunohistochemistry
Cochlea was collected from rats after injecting a lethal dose (1 ml) of Fatal-plus (Vortech Pharmaceuticals, Dearborn, MI) and transcardial perfusion with 0.1 M PBS and 10% formalin. The cochlea was postfixated in formalin, decalcified with 10% EDTA, and cryosectioned (40–50 μm) after an overnight incubation in 30% sucrose. The sections were blocked and incubated in anti-S-Nitroso-Cysteine (1:200) for 2 h followed by 1 h incubation in biotinylated secondary antibody (Vector Laboratories, Burlingame, CA). The sections were then treated with avidin and biotin (Elite ABC-kit; Vector Laboratories) to facilitate immunodetection with 0.05% DAB stain (7).
Abbreviations Used
- DAB
3′3′diaminobenzidine tetrahydrochloride
- DPOAE
distortion product otoacoustic emission
- NO
nitric oxide
- OC
organ of Corti
- OHC
outer hair cell
- SG
spiral ganglion
- SV
stria vascularis
Acknowledgments
This study has been funded by the National Institute on Deafness and other Communication Disorders, National Institutes of Health (5R03DC010225-03).
References
- 1.Benhar M. Stamler JS. A central role for S-nitrosylation in apoptosis. Nat Cell Biol. 2005;7:645–646. doi: 10.1038/ncb0705-645. [DOI] [PubMed] [Google Scholar]
- 2.Chanvorachote P. Nimmannit U. Stehlik C. Wang L. Jiang BH. Ongpipatanakul B. Rojanasakul Y. Nitric oxide regulates cell sensitivity to cisplatin-induced apoptosis through S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res. 2006;66:6353–6360. doi: 10.1158/0008-5472.CAN-05-4533. [DOI] [PubMed] [Google Scholar]
- 3.Foster MW. Hess DT. Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernlund E. Kutuk O. Basaga H. Linder S. Panaretakis T. Shoshan M. Cisplatin-induced nitrosylation of p53 prevents its mitochondrial translocation. Free Radic Biol Med. 2009;46:1607–1613. doi: 10.1016/j.freeradbiomed.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Hess DT. Matsumoto A. Kim SO. Marshall HE. Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 6.Jamesdaniel S. Ding D. Kermany MH. Davidson BA. Knight PR., 3rd Salvi R. Coling DE. Proteomic analysis of the balance between survival and cell death responses in cisplatin-mediated ototoxicity. J Proteome Res. 2008;7:3516–3524. doi: 10.1021/pr8002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamesdaniel S. Coling D. Hinduja S. Ding D. Li J. Cassidy L. Seigel M. Qu J. Salvi R. Cisplatin-induced ototoxicity is mediated by nitroxidative modification of cochlear proteins characterized by nitration of Lmo4. J Biol Chem. 2012 doi: 10.1074/jbc.M111.297960. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mannick JB. Schonhoff CM. Nitrosylation: the next phosphorylation? Arch Biochem Biophys. 2002;408:1–6. doi: 10.1016/s0003-9861(02)00490-3. [DOI] [PubMed] [Google Scholar]
- 9.Rybak LP. Whitworth CA. Mukherjea D. Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]