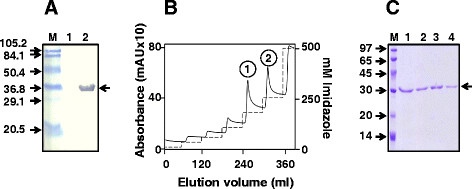
Figure 2.
Affinity purification of the recombinant HBcAg-EDIII-2 protein under denaturing conditions. (A) Western blot analysis of localization of HBcAg-EDIII-2 expression. Induced cells were sonicated and centrifuged. The resultant supernatant (lane 1) and pellet (lane 2) fractions were boiled in Laemmli loading buffer, electrophoresced under denaturing conditions and subjected to immunoblot analysis using mAb24A12 to identify the recombinant HBcAg-EDIII-2 protein. (B) Ni2+-affinity purification of HBcAg-EDIII-2 from induced E. coli cells. The insoluble pellet obtained after sonication of induced cells was purified using Ni2+-Sepharose under denaturing conditions. The solid curve represents the chromatographic profile obtained by measurement of absorbance at 280 nm. The two peaks discernible in the elution profile are numbered 1 and 2. The dotted curve represents the imidazole step-gradient employed for elution. (C) SDS-PAGE analysis of fractions corresponding to peaks 1 (lanes 1 & 2) and 2 (lanes 3 & 4) shown in panel ‘B’. Low molecular weight protein markers were run in lane ‘M’; their sizes (in kDa) are indicated to the left of the panel. The arrow at the right of the panels A and C indicates the position of the HBcAg-EDIII-2 protein.