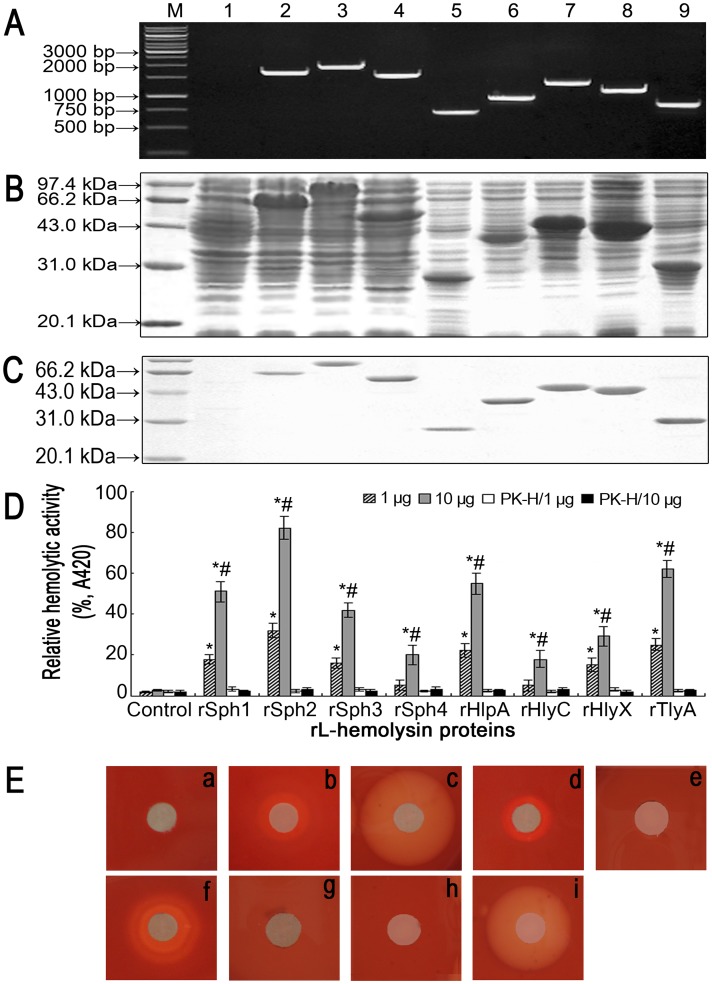
Figure 2. Expression, purification and hemolytic activity of rL-hemolysin proteins.
(A). Hemolysin genes amplified from genomic DNA of L. interrogans strain Lai. Lane AM: DNA marker (Fermentas, Canada). Lane A1: blank control. Lanes A2 to A9: amplicons of the sph1 (1674 bp), sph2 (1869 bp), sph3 (1557 bp), sph4 (717 bp), hlpA (939 bp), hlyC (1332 bp), hlyX (1176 bp) and tlyA (828 bp) genes from L. interrogans strain Lai. (B). Expression of the rL-hemolysin proteins. Lane BM: protein marker (Sangon Biotech, China). Lane B1: wild-type pET42a. Lanes B2 to B9: expressed recombinant proteins of rSph1 (63.5 kDa), rSph2 (71.1 kDa), rSph3 (60.7 kDa), rSph4 (27.9 kDa), rHlpA (36.5 kDa), rHlyC (50.4 kDa), rHlyX (43.1 kDa) and rTlyA (31.5 kDa). (C). Purification of the rL-hemolysin proteins. Lane CM: protein marker (Sangon Biotech). Lane C1: blank control. Lanes C2 to C9: purified rSph1, rSph2, rSph3, rSph4, rHlpA, rHlyC, rHlyX and rTlyA proteins. (D). Hemolytic activity of the rL-hemolysin proteins measured by spectrophotometry. Bars show the mean ± SD of three independent experiments. PK-H indicates that the rL-hemolysins were pretreated with proteinase K (PK) digestion plus heat-inactivation. The A420 values from spectrophotometric measurement at 420 nm reflect the levels of hemoglobin released from sheep erythrocytes. The A420 value of the supernatant from the sheep erythrocytes in 1 mL 5% erythrocyte suspension lysed by distilled water was set at 100% (total hemolysis). rOmpL1, a non-hemolytic recombinant porin from L. interrogans strain Lai, was the negative control (background hemolysis). Relative hemolytic activity of each of the rL-hemolysin proteins was defined as the percentage (%, A420) compared to total hemolysis. *P<0.05 vs relative hemolytic activity of leptospiral rOmpL1 protein, the negative control, at the same protein concentrations. # P<0.05 vs the relative hemolytic activity of 1 µg of the corresponding rL-hemolysin protein.(E). Hemolytic rings on sheep blood agar plates caused by the rL-hemolysin proteins. a: negative control containing 10 µg rOmpL1, a non-hemolytic recombinant porin from L. interrogans strain Lai. b to i: hemolytic rings caused by the rL-hemolysin proteins rSph1, rSph2, rSph3, rSph4, rHlyC, rHlyX, rHlpA and rTlyA.