Abstract
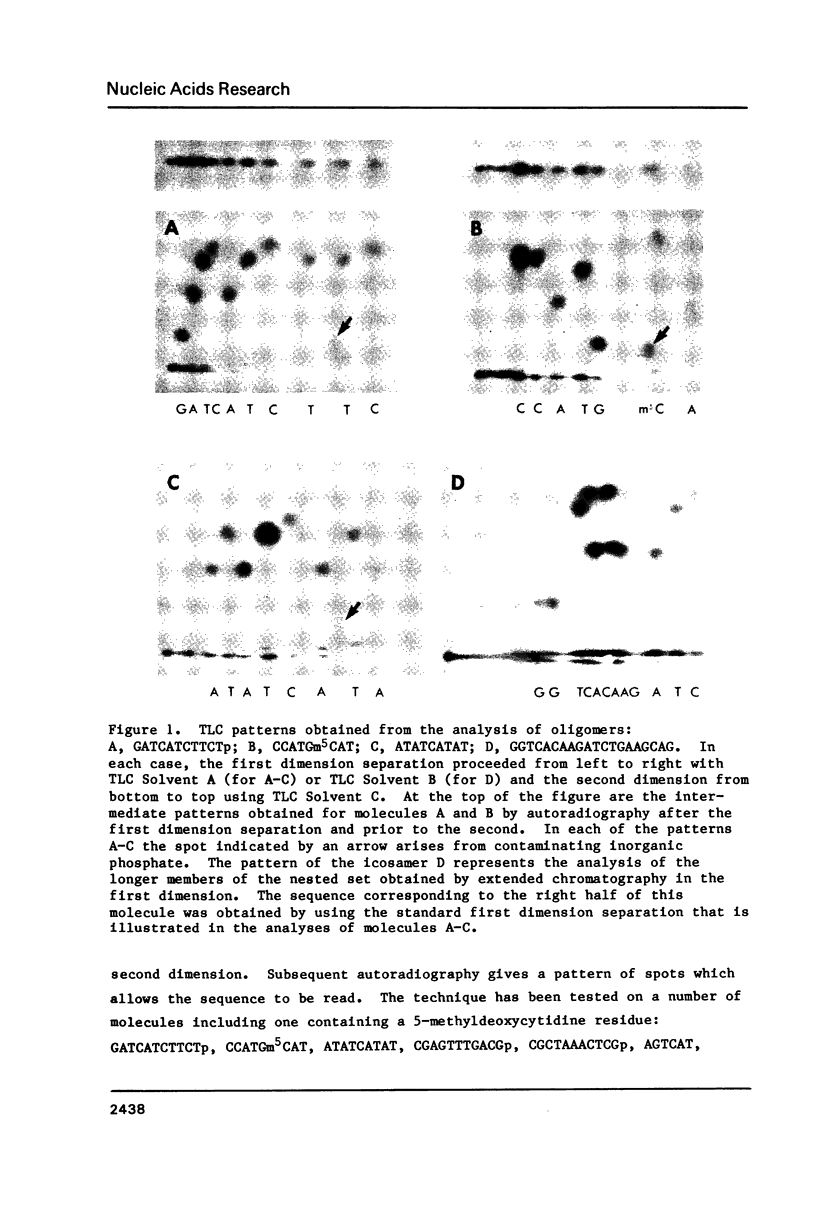
The structure of an oligodeoxyribonucleotide may be determined by a simple two-dimensional separation on a polyethyleneimine-cellulose thin layer sheet. Chromatography in the first dimension fractionates by chain length a nested set of fragments that are generated by subjecting the oligomer to partial spleen phosphodiesterase degradation and then labelling their non-common ends with 32P using polynucleotide kinase. A subsequent in situ treatment with nuclease Bal 31 produces labelled mononucleotides, and these are identified by chromatography in the second dimension. Since the method does not identify the 3' terminal nucleotide, a convenient procedure involving 3' end labelling followed by enzymatic digestion to monomers has been developed for this purpose. This approach to sequence analysis also has the advantage of permitting assignment of the identity and location of any modified or unusual bases within the oligonucleotide.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banaszuk A. M., Deugau K. V., Sherwood J., Michalak M., Glick B. R. An efficient method for the sequence analysis of oligodeoxyribonucleotides. Anal Biochem. 1983 Feb 1;128(2):281–286. doi: 10.1016/0003-2697(83)90376-7. [DOI] [PubMed] [Google Scholar]
- Bernardi A. A fast method of analysis of the 5' terminal nucleotides of deoxyribooligonucleotides. Anal Biochem. 1974 Jun;59(2):501–507. doi: 10.1016/0003-2697(74)90303-0. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- GRIFFIN B. E., REESE C. B. The synthesis of N1- and N6- methyladenosine 5'-pyrophosphates. Possible substrates for polynucleotide phosphorylase. Biochim Biophys Acta. 1963 Feb 26;68:185–192. doi: 10.1016/0006-3002(63)90134-3. [DOI] [PubMed] [Google Scholar]
- Gough G. R., Collier K. J., Weith H. L., Gilham P. T. The use of barium salts of protected deoxyribonucleoside-3' p-chlorophenyl phosphates for construction of oligonucleotides by the phosphotriester method: high-yield synthesis of dinucleotide blocks. Nucleic Acids Res. 1979 Dec 11;7(7):1955–1964. doi: 10.1093/nar/7.7.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough G. R., Singleton C. K., Weith H. L., Gilham P. T. Protected deoxyribonucleoside-3' aryl phosphodiesters as key intermediates in polynucleotide synthesis. Construction of an icosanucleotide analogous to the sequence at the ends of Rous sarcoma virus 35S RNA. Nucleic Acids Res. 1979 Apr;6(4):1557–1570. doi: 10.1093/nar/6.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E., Chia L. S., Gupta R. C., Sivarajan M. Sequence analysis of nonradioactive RNA fragments by periodate-phosphatase digestion and chemical tritium labeling: characterization of large oligonucleotides and oligonucleotides containing modified nucleosides. Nucleic Acids Res. 1974 Sep;1(9):1121–1141. doi: 10.1093/nar/1.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Jay E., Bahl C. P., Wu R. A reliable mapping method for sequence determination of oligodeoxyribonucleotides by mobility shift analysis. Anal Biochem. 1976 Jul;74(1):73–93. doi: 10.1016/0003-2697(76)90311-0. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Wu R. Sequence analysis of short DNA fragments. Methods Enzymol. 1980;65(1):620–638. [PubMed] [Google Scholar]
- Wu R., Tu C. D., Padmanabhan R. Nucleotide sequence analysis of DNA. XII. The chemical synthesis and sequence analysis of a dodecadeoxynucleotide which binds to the endolysin gene of bacteriophage lambda. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1092–1099. doi: 10.1016/s0006-291x(73)80007-5. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Kleppe K., Khorana H. G. Reversal of bacteriophage T4 induced polynucleotide kinase action. Biochemistry. 1973 Dec 4;12(25):5050–5055. doi: 10.1021/bi00749a004. [DOI] [PubMed] [Google Scholar]